content review for prerequisite validation - laccd
... 11. Calculate equilibrium constants for reactions and predict the effect of reaction conditions and concentrations on the position of equilibrium. 12. Interpret simple reaction profiles and their relationships to reaction mechanism; describe relationship between activation energy of an elementary re ...
... 11. Calculate equilibrium constants for reactions and predict the effect of reaction conditions and concentrations on the position of equilibrium. 12. Interpret simple reaction profiles and their relationships to reaction mechanism; describe relationship between activation energy of an elementary re ...
Alkene Addition Reactions
... (CH3-‐). The order of migrating groups is H > CH3. Alcohols can be produced by addition of water around the double bond in the process called hydration. This is accomplished by using an ...
... (CH3-‐). The order of migrating groups is H > CH3. Alcohols can be produced by addition of water around the double bond in the process called hydration. This is accomplished by using an ...
Visualizing the Transition State and
... HOW THE DEMONSTRATION ADDRESSES THE CHEMICAL CONCEPTS Transition state theory (TST), also called activated complex theory, is often introduced in general chemistry courses when discussing kinetics. A reaction energy diagram is used to follow the progress of the reaction from reactants through a tran ...
... HOW THE DEMONSTRATION ADDRESSES THE CHEMICAL CONCEPTS Transition state theory (TST), also called activated complex theory, is often introduced in general chemistry courses when discussing kinetics. A reaction energy diagram is used to follow the progress of the reaction from reactants through a tran ...
Formula Mass (weight)
... • What important law is adhered to by this reaction (and all the reactions we will study in CHEM 111) ...
... • What important law is adhered to by this reaction (and all the reactions we will study in CHEM 111) ...
06 MC /08 MC /08 NMR
... only trans-l-4-dimethylcyclohexane. onlycrs-1-4-dimethylcyclohexane. both trans and cis-l -4-dimethylcyclohexane. It's impossible to tell. --'i ...
... only trans-l-4-dimethylcyclohexane. onlycrs-1-4-dimethylcyclohexane. both trans and cis-l -4-dimethylcyclohexane. It's impossible to tell. --'i ...
Organic Tutorial 1st Year HT01
... consider Michael addition and the factors which determine 1,2 or 1,4 addition ...
... consider Michael addition and the factors which determine 1,2 or 1,4 addition ...
Form A 1 Chem 130 Name______________________________
... Comparing reactions 1 and 2: [A] remains constant, but [B] changes by (0.0370/0.0185) = 2 times. The rate changes by (6.75 x 10-4/3.35 x 10-4) = 2.01 times. Since the change in rate is the same as the change in concentration, the reaction must be first order in B. Now comparing reactions 2 and 3: [B ...
... Comparing reactions 1 and 2: [A] remains constant, but [B] changes by (0.0370/0.0185) = 2 times. The rate changes by (6.75 x 10-4/3.35 x 10-4) = 2.01 times. Since the change in rate is the same as the change in concentration, the reaction must be first order in B. Now comparing reactions 2 and 3: [B ...
Lab 2 - Academic Computer Center
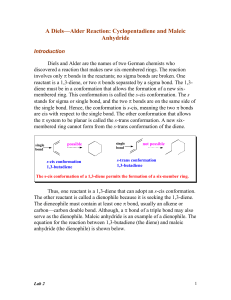
... Typical Dienophiles for Diels-Alder Reactions How does one recognize a Diels-Alder reaction? These reactions are easy to spot, because they involve two organic reactants and only heat as a reactant. One of the reactants (the diene) must have a conjugated diene system, and the other reactant (the die ...
... Typical Dienophiles for Diels-Alder Reactions How does one recognize a Diels-Alder reaction? These reactions are easy to spot, because they involve two organic reactants and only heat as a reactant. One of the reactants (the diene) must have a conjugated diene system, and the other reactant (the die ...
Rates of Reaction: Chemical Kinetics 50
... adjacent office to telephone her agent in New York City. The agent has her secretary write a note to Dr. Goodall. The note is typed and faxed to Africa. The fax is placed in an envelope when it is received and given to a messenger who must travel a few kilometres by boat and a few hundred metres on ...
... adjacent office to telephone her agent in New York City. The agent has her secretary write a note to Dr. Goodall. The note is typed and faxed to Africa. The fax is placed in an envelope when it is received and given to a messenger who must travel a few kilometres by boat and a few hundred metres on ...
Microsoft Word - Final Exam Study Guide
... stability, elimination reactions, Zaitsev’s rule, E1 mechanism, E2 mechanism, antiperiplanar, comparing substitution and elimination mechanisms, synthesis of ethers, alcohols, and epoxides, dehydration of alcohols, carbocation rearrangements, reactions of alcohols/ethers/epoxides, multistep synthesi ...
... stability, elimination reactions, Zaitsev’s rule, E1 mechanism, E2 mechanism, antiperiplanar, comparing substitution and elimination mechanisms, synthesis of ethers, alcohols, and epoxides, dehydration of alcohols, carbocation rearrangements, reactions of alcohols/ethers/epoxides, multistep synthesi ...
Chapter 9
... Three (or more) atom molecules cannot be explained by simple overlap of orbitals. Fact: a bond generally forms between two half-filled orbitals. Fact: an s-type orbital is spherical, so it could form a bond in any direction. Fact: the three p-type orbitals are at 90 degree angles to each other. ...
... Three (or more) atom molecules cannot be explained by simple overlap of orbitals. Fact: a bond generally forms between two half-filled orbitals. Fact: an s-type orbital is spherical, so it could form a bond in any direction. Fact: the three p-type orbitals are at 90 degree angles to each other. ...
Topic 16 Assessed Homework - A
... State what you would observe when propanoic acid reacts with this reagent. Reagent ............................................................................................. Observation ....................................................................................... ...
... State what you would observe when propanoic acid reacts with this reagent. Reagent ............................................................................................. Observation ....................................................................................... ...
KEY Final Exam Review - Iowa State University
... NO3¯ ---> NO 2) balance each half-reaction: 8H2S ---> S8 + 16H+ + 16e¯ 3e¯ + 4H+ + NO3¯ ---> NO + 2H2O 3) Make the number of electrons equal: 24H2S ---> 3S8 + 48H+ + 48e¯ <--- multiplied by a factor of 3 48e¯ + 64H+ + 16NO3¯ ---> 16NO + 32H2O <--- multiplied by a factor of 16 Note that 16 and 3 have ...
... NO3¯ ---> NO 2) balance each half-reaction: 8H2S ---> S8 + 16H+ + 16e¯ 3e¯ + 4H+ + NO3¯ ---> NO + 2H2O 3) Make the number of electrons equal: 24H2S ---> 3S8 + 48H+ + 48e¯ <--- multiplied by a factor of 3 48e¯ + 64H+ + 16NO3¯ ---> 16NO + 32H2O <--- multiplied by a factor of 16 Note that 16 and 3 have ...
Ethers, Sulfides, Epoxides
... Ok, what to protonate? Several oxygens and the double bond. Protonation of an alcohol can set-up a better leaving group. Protonation of a carbonyl can create a ...
... Ok, what to protonate? Several oxygens and the double bond. Protonation of an alcohol can set-up a better leaving group. Protonation of a carbonyl can create a ...
Woodward–Hoffmann rules

The Woodward–Hoffmann rules, devised by Robert Burns Woodward and Roald Hoffmann, are a set of rules in organic chemistry predicting the barrier heights of pericyclic reactions based upon conservation of orbital symmetry. The Woodward–Hoffmann rules can be applied to understand electrocyclic reactions, cycloadditions (including cheletropic reactions), sigmatropic reactions, and group transfer reactions. Reactions are classified as allowed if the electronic barrier is low, and forbidden if the barrier is high. Forbidden reactions can still take place but require significantly more energy.The Woodward–Hoffmann rules were first formulated to explain the striking stereospecificity of electrocyclic reactions under thermal and photochemical control. Thermolysis of the substituted cyclobutene trans-1,2,3,4-tetramethylcyclobutene (1) gave only one diastereomer, the (E,E)-3,4-dimethyl-2,4-hexadiene (2) as shown below; the (Z,Z) and the (E,Z) diastereomers were not detected in the reaction. Similarly, thermolysis of cis-1,2,3,4-tetramethylcyclobutene (3) gave only the (E,Z) diastereomer (4).Due to their elegance and simplicity, the Woodward–Hoffmann rules are credited with first exemplifying the power of molecular orbital theory to experimental chemists. Hoffmann was awarded the 1981 Nobel Prize in Chemistry for this work, shared with Kenichi Fukui who developed a similar model using frontier molecular orbital (FMO) theory; because Woodward had died two years before, he was not eligible to win what would have been his second Nobel Prize for Chemistry.