Download
... 18. Phenol is heated with phthalic anhydride in the presence of concentrated The product is (a) Bakelite (b)Fluorescein (c)Salicylicacid (d) Phenolphthalein 19. Phenol on distillation with zinc dust gives (a) C6 H6 (b) C6 H12 (c) C6 H 5 OC6 H 5 (d) C6 H5 − C6 H5 20. Phenol is converted into salicyla ...
... 18. Phenol is heated with phthalic anhydride in the presence of concentrated The product is (a) Bakelite (b)Fluorescein (c)Salicylicacid (d) Phenolphthalein 19. Phenol on distillation with zinc dust gives (a) C6 H6 (b) C6 H12 (c) C6 H 5 OC6 H 5 (d) C6 H5 − C6 H5 20. Phenol is converted into salicyla ...
Nucleophilic Substitution Reactions
... ■ The polarity in halogenoalkanes is due to the fact that the halogen atom is more electronegative than carbon, and so exserts a stronger pull on the shared electrons in the carbon-halogen bond. ■ As a result, the halogen gains a partial negative charge and the carbon gains a partial positive charge ...
... ■ The polarity in halogenoalkanes is due to the fact that the halogen atom is more electronegative than carbon, and so exserts a stronger pull on the shared electrons in the carbon-halogen bond. ■ As a result, the halogen gains a partial negative charge and the carbon gains a partial positive charge ...
aldehyde,ketones and Haloalkanes
... Q.1 Draw the structure of the compound whose IUPAC name is 4 – chloro-1-phenylpentane – 2 –one.[1] Q.2 Write the IUPAC name of the following compound: (CH3)3CCH2Br. [1] Q.3 Arrange the compound of each set in order of reactivity towards SN2 displacement. 1 – Bromo – 3 – methylbutane , 2 – Bromo – 2 ...
... Q.1 Draw the structure of the compound whose IUPAC name is 4 – chloro-1-phenylpentane – 2 –one.[1] Q.2 Write the IUPAC name of the following compound: (CH3)3CCH2Br. [1] Q.3 Arrange the compound of each set in order of reactivity towards SN2 displacement. 1 – Bromo – 3 – methylbutane , 2 – Bromo – 2 ...
REDOX REACTIONS IN ORGANIC CHEMISTRY
... Balancing Redox Equations: In order to calculate the theoretical and actual yield from a reaction, a balanced chemical equation is necessary. The following rules describe the Ion-Electron Half-Reaction Method for balancing redox equations ... 1. Break the equation into 2 half-reactions, i.e., oxidat ...
... Balancing Redox Equations: In order to calculate the theoretical and actual yield from a reaction, a balanced chemical equation is necessary. The following rules describe the Ion-Electron Half-Reaction Method for balancing redox equations ... 1. Break the equation into 2 half-reactions, i.e., oxidat ...
Important Concepts from Chapter 9 • DRAWING LEWIS ELECTRON
... Orbital hybridization theory explains bonding in molecules with more than two atoms. A new set of hybrid orbitals can be created by mixing the s, p, and d orbitals on an atom. 1. The number of hybrid orbitals is always the __________ as the number of atomic orbitals mixed to create them 2. The hybr ...
... Orbital hybridization theory explains bonding in molecules with more than two atoms. A new set of hybrid orbitals can be created by mixing the s, p, and d orbitals on an atom. 1. The number of hybrid orbitals is always the __________ as the number of atomic orbitals mixed to create them 2. The hybr ...
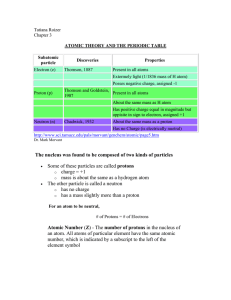
atomic theory and the periodic table
... Each orbital has a name. The orbital occupied by the hydrogen electron is called a 1s orbital. The "1" represents the fact that the orbital is in the energy level closest to the nucleus. The "s" tells you about the shape of the orbital. s orbitals are spherically symmetric around the nucleus - in e ...
... Each orbital has a name. The orbital occupied by the hydrogen electron is called a 1s orbital. The "1" represents the fact that the orbital is in the energy level closest to the nucleus. The "s" tells you about the shape of the orbital. s orbitals are spherically symmetric around the nucleus - in e ...
Document
... Ionic Bonding Ionic bonds are common in inorganic chemistry but rare in organic chemistry. Carbon shows less of a tendency to form cations than metals do, and less of a tendency to form anions than nonmetals. ...
... Ionic Bonding Ionic bonds are common in inorganic chemistry but rare in organic chemistry. Carbon shows less of a tendency to form cations than metals do, and less of a tendency to form anions than nonmetals. ...
Chapter 5. An Overview of Organic Reactions
... the (conceptual) transition-state structure for the first step The bond between ...
... the (conceptual) transition-state structure for the first step The bond between ...
Today Electrochemistry electrons moving about equilibrium with a
... Keeping track of charge Easy in ions "Book keeping" in molecules for molecules oxidation numbers are a convention in which we imagine what the charge would be if it broke up into ionic pieces (we can't really assign electrons to different elements) ...
... Keeping track of charge Easy in ions "Book keeping" in molecules for molecules oxidation numbers are a convention in which we imagine what the charge would be if it broke up into ionic pieces (we can't really assign electrons to different elements) ...
Today Electrochemistry electrons moving about equilibrium with a
... "Book keeping" in molecules! for molecules oxidation numbers are a convention ! in which we imagine what the ! charge would be if it broke up into ionic pieces! (we can't really assign electrons to different elements)! ...
... "Book keeping" in molecules! for molecules oxidation numbers are a convention ! in which we imagine what the ! charge would be if it broke up into ionic pieces! (we can't really assign electrons to different elements)! ...
9. E1: Alkenes from alcohols - Web Pages
... You will perform two alkene tests to determine whether or not you have the alkene product instead of the alkane starting material or some other non-alkene impurity. Alkenes decolorize the iodine and potassium permanganate solutions you will be using. A positive test for the presence of an alkene in ...
... You will perform two alkene tests to determine whether or not you have the alkene product instead of the alkane starting material or some other non-alkene impurity. Alkenes decolorize the iodine and potassium permanganate solutions you will be using. A positive test for the presence of an alkene in ...
Hydrogen Production by Splitting Water in an Electrolyzer
... reaction, and the characteristic rate of each step. In most studies, this information is obtained by experimental analysis. After carrying out an experiments and analyzing the results, a mechanism is written down. This modeling of the process can be used not only to describe the experimental results ...
... reaction, and the characteristic rate of each step. In most studies, this information is obtained by experimental analysis. After carrying out an experiments and analyzing the results, a mechanism is written down. This modeling of the process can be used not only to describe the experimental results ...
SMJK PEREMPUAN CHINA PULAU PINANG CHEMISTRY FORM 5
... (ii) Write the thermochemical equation for the combustion of propanol. Then, calculate the volume of carbon dioxide that is released at room conditions when 1.2 g of propanol is burned in excess oxygen in the laboratory. [Relative atomic mass: H,1; C,12; O,16; Molar volume of gas = 24dm3 mol-1 at ro ...
... (ii) Write the thermochemical equation for the combustion of propanol. Then, calculate the volume of carbon dioxide that is released at room conditions when 1.2 g of propanol is burned in excess oxygen in the laboratory. [Relative atomic mass: H,1; C,12; O,16; Molar volume of gas = 24dm3 mol-1 at ro ...
A Guide to Rate of Reactions
... It is important to note that the CAPS document separates Rate of Reaction and Chemical Equilibrium. This is because the underlying theory of each of these is very different. Rate of reaction is also called Chemical Kinetics and deals with how fast a reaction happens. Chemical equilibrium is based on ...
... It is important to note that the CAPS document separates Rate of Reaction and Chemical Equilibrium. This is because the underlying theory of each of these is very different. Rate of reaction is also called Chemical Kinetics and deals with how fast a reaction happens. Chemical equilibrium is based on ...
Bonding
... Ionic Bonding Ionic bonds are common in inorganic chemistry but rare in organic chemistry. Carbon shows less of a tendency to form cations than metals do, and less of a tendency to form anions than nonmetals. ...
... Ionic Bonding Ionic bonds are common in inorganic chemistry but rare in organic chemistry. Carbon shows less of a tendency to form cations than metals do, and less of a tendency to form anions than nonmetals. ...
Woodward–Hoffmann rules

The Woodward–Hoffmann rules, devised by Robert Burns Woodward and Roald Hoffmann, are a set of rules in organic chemistry predicting the barrier heights of pericyclic reactions based upon conservation of orbital symmetry. The Woodward–Hoffmann rules can be applied to understand electrocyclic reactions, cycloadditions (including cheletropic reactions), sigmatropic reactions, and group transfer reactions. Reactions are classified as allowed if the electronic barrier is low, and forbidden if the barrier is high. Forbidden reactions can still take place but require significantly more energy.The Woodward–Hoffmann rules were first formulated to explain the striking stereospecificity of electrocyclic reactions under thermal and photochemical control. Thermolysis of the substituted cyclobutene trans-1,2,3,4-tetramethylcyclobutene (1) gave only one diastereomer, the (E,E)-3,4-dimethyl-2,4-hexadiene (2) as shown below; the (Z,Z) and the (E,Z) diastereomers were not detected in the reaction. Similarly, thermolysis of cis-1,2,3,4-tetramethylcyclobutene (3) gave only the (E,Z) diastereomer (4).Due to their elegance and simplicity, the Woodward–Hoffmann rules are credited with first exemplifying the power of molecular orbital theory to experimental chemists. Hoffmann was awarded the 1981 Nobel Prize in Chemistry for this work, shared with Kenichi Fukui who developed a similar model using frontier molecular orbital (FMO) theory; because Woodward had died two years before, he was not eligible to win what would have been his second Nobel Prize for Chemistry.