Here is the Original File - University of New Hampshire
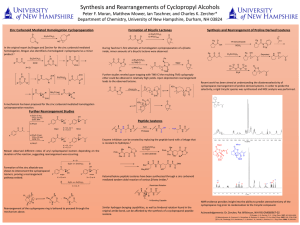
... Further studies reveled upon trapping with TMS-Cl the resulting TMS cycloproply ether could be obtained in relatively high yields. Upon deprotection rearrangement leads to the observed lactone. ...
... Further studies reveled upon trapping with TMS-Cl the resulting TMS cycloproply ether could be obtained in relatively high yields. Upon deprotection rearrangement leads to the observed lactone. ...
organic revision nots
... 6. The boiling points of aldehydes and ketones are higher than hydrocarbons and ethers of comparable molecular masses. 7. Why is there a large difference in the boiling points of butanal and butan-1-ol? 8. The lower members of aldehydes and ketones such as methanal,ethanal and propanone are miscible ...
... 6. The boiling points of aldehydes and ketones are higher than hydrocarbons and ethers of comparable molecular masses. 7. Why is there a large difference in the boiling points of butanal and butan-1-ol? 8. The lower members of aldehydes and ketones such as methanal,ethanal and propanone are miscible ...
Midterm 2 Review slides from November 15
... valence electrons are in the localized atomic orbitals of isolated atoms these are the s,p,d,f ,p, , orbitals bond is formed from overlap of half-filled valence orbitals, spin-pairing of valence electrons if the interactions ...
... valence electrons are in the localized atomic orbitals of isolated atoms these are the s,p,d,f ,p, , orbitals bond is formed from overlap of half-filled valence orbitals, spin-pairing of valence electrons if the interactions ...
Chemical Equilibrium II
... _________________ for gases (although for the purposes of Chemistry 12, we will not be using partial pressures) Some rules to follow when writing equilibrium expressions: “____________” concentrations in equilibrium expressions: Substances whose concentrations undergo no significant change in a chem ...
... _________________ for gases (although for the purposes of Chemistry 12, we will not be using partial pressures) Some rules to follow when writing equilibrium expressions: “____________” concentrations in equilibrium expressions: Substances whose concentrations undergo no significant change in a chem ...
Required Resources and Materials
... (Each team is labeled with 1 model kit and access to boards or large sheet with markers) Split class into even numbered groups (Counting off to 6) Team will delegate one presenter and the rest will be research associates 6 groups: Names – Faraday, Planck, Bohr, Huckel, Avagadro and Hess Make ...
... (Each team is labeled with 1 model kit and access to boards or large sheet with markers) Split class into even numbered groups (Counting off to 6) Team will delegate one presenter and the rest will be research associates 6 groups: Names – Faraday, Planck, Bohr, Huckel, Avagadro and Hess Make ...
SF Chemical Kinetics Michaelmas 2011 L5
... • The theoretical approach is based on the kinetic theory of gases. • Molecules are assumed to be hard structureless spheres. Hence the model neglects the discrete chemical structure of an individual molecule. This assumption is unrealistic. • We also assume that no interaction between molecules unt ...
... • The theoretical approach is based on the kinetic theory of gases. • Molecules are assumed to be hard structureless spheres. Hence the model neglects the discrete chemical structure of an individual molecule. This assumption is unrealistic. • We also assume that no interaction between molecules unt ...
Honors Chemistry 2 Chapter 10 Test Review
... d. From part C, calculate how much heat is released when 100 grams of calcium oxide react with liquid water to form calcium hydroxide. ...
... d. From part C, calculate how much heat is released when 100 grams of calcium oxide react with liquid water to form calcium hydroxide. ...
aldehydes and ketones
... • The carbonyl carbon of an aldehyde is more accessible to the nucleophile because the hydrogen attached to the carbonyl carbon of an aldehyde is smaller than the second alkyl group to carbonyl carbon of a ketone. • Ketones have greater steric crowding in their transition states, so they have less s ...
... • The carbonyl carbon of an aldehyde is more accessible to the nucleophile because the hydrogen attached to the carbonyl carbon of an aldehyde is smaller than the second alkyl group to carbonyl carbon of a ketone. • Ketones have greater steric crowding in their transition states, so they have less s ...
... is advantageous over the chemical ones, as they ensure the stereo-selective obtaining of the product. Although it has been shown that their chemical synthesis was well-developed, the method still employs toxic and expensive compounds to yield α and β-configuration products via several steps. Hence, ...
Enantioselective Henry Reactions under Dual Lewis Acid/Amine
... selectivity (entry 1), whereas increasing the ligand loading above 45 mol % did not improve the result. The quantity of iPr2EtN was crucial too. Lower loading or absence of iPr2EtN (entries 3 and 4) led to diminished yields and ee values. Interestingly, the absence of iPr2EtN could be partially comp ...
... selectivity (entry 1), whereas increasing the ligand loading above 45 mol % did not improve the result. The quantity of iPr2EtN was crucial too. Lower loading or absence of iPr2EtN (entries 3 and 4) led to diminished yields and ee values. Interestingly, the absence of iPr2EtN could be partially comp ...
resonance effects - HCC Learning Web
... an intermediate benzylic radical Reacts with Br2 to yield product Br· radical cycles back into reaction to carry chain Br2 produced from reaction of HBr with NBS ...
... an intermediate benzylic radical Reacts with Br2 to yield product Br· radical cycles back into reaction to carry chain Br2 produced from reaction of HBr with NBS ...
Acid-Catalyzed Dehydration of Alcohols
... as a layer on the surface of the acid-alcohol mixture (the density of the alkene is less than one); the gas can then be collected over water or the liquid layer can be removed by simple distillation to give the final alkene product. In alcohols where there are more than two kinks of -hydrogens, the ...
... as a layer on the surface of the acid-alcohol mixture (the density of the alkene is less than one); the gas can then be collected over water or the liquid layer can be removed by simple distillation to give the final alkene product. In alcohols where there are more than two kinks of -hydrogens, the ...
Covalent Bonding
... to form hybrid orbitals • Atomic orbitals involved in bonding often contain a single unpaired electron • When the orbitals hybridize, a pair of electrons is shared • These hybrid orbitals are equal in number to the atomic orbitals which made them ...
... to form hybrid orbitals • Atomic orbitals involved in bonding often contain a single unpaired electron • When the orbitals hybridize, a pair of electrons is shared • These hybrid orbitals are equal in number to the atomic orbitals which made them ...
A Biocatalytic Henry Reaction-The Hydroxynitrile Lyase from Hevea
... have to await results from proper kinetics experiments, the reduced yield obtained for the addition of [1,1-D2]nitroethane to benzaldehyde already indicates the existence of a kinetic isotope effect and suggests the deprotonation of the nitroalkane to be the rate-limiting step. In contrast, in the H ...
... have to await results from proper kinetics experiments, the reduced yield obtained for the addition of [1,1-D2]nitroethane to benzaldehyde already indicates the existence of a kinetic isotope effect and suggests the deprotonation of the nitroalkane to be the rate-limiting step. In contrast, in the H ...
Ethers, Sulfides, Epoxides - City University of New York
... Generally, the hemiacetals and acetals are only a minor component of an equilibrium mixture. In order to favor formation of acetals the carbonyl compound and alcohol is reacted with acid in the absence of water. Dry HCl) The acetals or hemiacetals maybe converted back to the carbonyl compound by tre ...
... Generally, the hemiacetals and acetals are only a minor component of an equilibrium mixture. In order to favor formation of acetals the carbonyl compound and alcohol is reacted with acid in the absence of water. Dry HCl) The acetals or hemiacetals maybe converted back to the carbonyl compound by tre ...
Experiment 11 CHEMICAL REACTIONS
... Put a very small amount of solid copper (II) carbonate into your crucible and warm gently for one minute, then heat strongly for an additional 3 minutes. Observation: ______________ _____________________________________________________ Reaction Equation: ...
... Put a very small amount of solid copper (II) carbonate into your crucible and warm gently for one minute, then heat strongly for an additional 3 minutes. Observation: ______________ _____________________________________________________ Reaction Equation: ...
Woodward–Hoffmann rules

The Woodward–Hoffmann rules, devised by Robert Burns Woodward and Roald Hoffmann, are a set of rules in organic chemistry predicting the barrier heights of pericyclic reactions based upon conservation of orbital symmetry. The Woodward–Hoffmann rules can be applied to understand electrocyclic reactions, cycloadditions (including cheletropic reactions), sigmatropic reactions, and group transfer reactions. Reactions are classified as allowed if the electronic barrier is low, and forbidden if the barrier is high. Forbidden reactions can still take place but require significantly more energy.The Woodward–Hoffmann rules were first formulated to explain the striking stereospecificity of electrocyclic reactions under thermal and photochemical control. Thermolysis of the substituted cyclobutene trans-1,2,3,4-tetramethylcyclobutene (1) gave only one diastereomer, the (E,E)-3,4-dimethyl-2,4-hexadiene (2) as shown below; the (Z,Z) and the (E,Z) diastereomers were not detected in the reaction. Similarly, thermolysis of cis-1,2,3,4-tetramethylcyclobutene (3) gave only the (E,Z) diastereomer (4).Due to their elegance and simplicity, the Woodward–Hoffmann rules are credited with first exemplifying the power of molecular orbital theory to experimental chemists. Hoffmann was awarded the 1981 Nobel Prize in Chemistry for this work, shared with Kenichi Fukui who developed a similar model using frontier molecular orbital (FMO) theory; because Woodward had died two years before, he was not eligible to win what would have been his second Nobel Prize for Chemistry.