Synthesis of enantiopure alcohols
... Previously we have reported that the enantioselectivity (E) decreased during esterifications of a range of secondary alcohols (1-4) catalyzed by immobilized lipase B from Candida antarctica (Novozym 435) and that addition of enantiopure (R)alcohols, (R)-1, (R)-2, (R)-5, (R)-6 and (R)-7, induced incr ...
... Previously we have reported that the enantioselectivity (E) decreased during esterifications of a range of secondary alcohols (1-4) catalyzed by immobilized lipase B from Candida antarctica (Novozym 435) and that addition of enantiopure (R)alcohols, (R)-1, (R)-2, (R)-5, (R)-6 and (R)-7, induced incr ...
Chapter 11 - Alcohols and Ethers1
... the bulky substituent is a protecting group of the hydroxyl group - Note: MTBE stands for methyl ...
... the bulky substituent is a protecting group of the hydroxyl group - Note: MTBE stands for methyl ...
Practice Problem - HCC Southeast Commons
... intermediate loses H+ to regenerate the aromatic ring and yield a substitution product in which H+ is replaced by Br+ – It is similar to the 2nd step of an E1 reaction – The carbocation intermediate transfers a H+ to FeBr4- (from Br- and FeBr3) – This restores aromaticity (in contrast with addition ...
... intermediate loses H+ to regenerate the aromatic ring and yield a substitution product in which H+ is replaced by Br+ – It is similar to the 2nd step of an E1 reaction – The carbocation intermediate transfers a H+ to FeBr4- (from Br- and FeBr3) – This restores aromaticity (in contrast with addition ...
chapter 9 Zumdahl
... The Molecular Orbital Model Bonding in Homonuclear Diatomic Molecules Bonding in Heteronuclear Diatomic Molecules Combining the Localized Electron and Molecular Orbital Models ...
... The Molecular Orbital Model Bonding in Homonuclear Diatomic Molecules Bonding in Heteronuclear Diatomic Molecules Combining the Localized Electron and Molecular Orbital Models ...
chapter 09
... The Molecular Orbital Model Bonding in Homonuclear Diatomic Molecules Bonding in Heteronuclear Diatomic Molecules Combining the Localized Electron and Molecular Orbital Models ...
... The Molecular Orbital Model Bonding in Homonuclear Diatomic Molecules Bonding in Heteronuclear Diatomic Molecules Combining the Localized Electron and Molecular Orbital Models ...
Chapter 9
... The Molecular Orbital Model Bonding in Homonuclear Diatomic Molecules Bonding in Heteronuclear Diatomic Molecules Combining the Localized Electron and Molecular Orbital Models ...
... The Molecular Orbital Model Bonding in Homonuclear Diatomic Molecules Bonding in Heteronuclear Diatomic Molecules Combining the Localized Electron and Molecular Orbital Models ...
Molecular Geometry and Hybridization
... Molecular geometry is the three-dimensional arrangement of atoms in a molecule. The arrangement of atoms in a molecule affects the physical and chemical properties of a molecule. The question is how to predict the threedimensional arrangement of atoms in a molecule? The answer involves an assumption ...
... Molecular geometry is the three-dimensional arrangement of atoms in a molecule. The arrangement of atoms in a molecule affects the physical and chemical properties of a molecule. The question is how to predict the threedimensional arrangement of atoms in a molecule? The answer involves an assumption ...
Exam 1 Review Sheet Chapter 15 Chemistry 110b
... Synthesis of ketones: Review those from last semester and chapter 15. Ketones from the reaction of nitriles with RMgX or RLi, know the mechanism of this reaction and identify the unstable imine intermediate. [10e, 738-739; 11e, 729731] ...
... Synthesis of ketones: Review those from last semester and chapter 15. Ketones from the reaction of nitriles with RMgX or RLi, know the mechanism of this reaction and identify the unstable imine intermediate. [10e, 738-739; 11e, 729731] ...
- Wiley Online Library
... the course of 2 min. A precipitate of LiCl formed. L-valine (2.34 g, 20 mmol) was added portionwise to the mixture within 5 min. After stirring for 24 h at room temperature, 30 mL of MeOH were cautiously added and the volatiles removed by distillation. The residue was treated with 20% KOH solution a ...
... the course of 2 min. A precipitate of LiCl formed. L-valine (2.34 g, 20 mmol) was added portionwise to the mixture within 5 min. After stirring for 24 h at room temperature, 30 mL of MeOH were cautiously added and the volatiles removed by distillation. The residue was treated with 20% KOH solution a ...
Synthesis of (−)-Epibatidine - David A. Evans
... Although the reduction proceeded without affecting the chloropyridine ring,21 a 75:25 mixture of inseparable alcohols was obtained. The structural assignment of the major diastereomer was complicated as a result of slowly interconverting conformations as observed by 1H NMR spectroscopy, even at elev ...
... Although the reduction proceeded without affecting the chloropyridine ring,21 a 75:25 mixture of inseparable alcohols was obtained. The structural assignment of the major diastereomer was complicated as a result of slowly interconverting conformations as observed by 1H NMR spectroscopy, even at elev ...
Alkane
... For RX compound, when X = Cl or F ,the b.p. will be higher than alkane which have the same molecular mass due to the dipole-dipole interaction between the molecules. Solubility As the interaction between water and RX are quite different, (H-bond and dipole-dipole),they are only sightly soluble in wa ...
... For RX compound, when X = Cl or F ,the b.p. will be higher than alkane which have the same molecular mass due to the dipole-dipole interaction between the molecules. Solubility As the interaction between water and RX are quite different, (H-bond and dipole-dipole),they are only sightly soluble in wa ...
Addition of H 2 O to an Alkene
... – 3°carbocation is more stable than a 2° carbocation, and requires a lower activation energy for its formation. – 2°carbocation is, in turn, more stable than a 1° carbocation, and requires a lower activation energy for its formation. – Methyl and 1°carbocations are so unstable that they are never ob ...
... – 3°carbocation is more stable than a 2° carbocation, and requires a lower activation energy for its formation. – 2°carbocation is, in turn, more stable than a 1° carbocation, and requires a lower activation energy for its formation. – Methyl and 1°carbocations are so unstable that they are never ob ...
Entering and leaving group effects in Oh ligand substitutions
... Ligands trans-to each other compete for electron density because both M-L bonds use the same metal orbitals, e.g. dz2 or pz See p. 831 in Ch.22, Box 22.8: Very strong -donors (e.g. H– and alkyl– ligands) compete for orbital overlap at the metal with the leaving group: this weakens the M-L bond in t ...
... Ligands trans-to each other compete for electron density because both M-L bonds use the same metal orbitals, e.g. dz2 or pz See p. 831 in Ch.22, Box 22.8: Very strong -donors (e.g. H– and alkyl– ligands) compete for orbital overlap at the metal with the leaving group: this weakens the M-L bond in t ...
A Direct Access to 3-(2-Oxoalkyl)indoles via
... Abstract: 3-Methylindole is acylated regioselectively at the methyl group when treated with a variety of acyl chlorides in 1,2-dichloroethane in the presence of AlCl3, affording a mild and direct method for the synthesis of 3-(2-oxoalkyl)indoles. The product formation in this one-pot reaction largel ...
... Abstract: 3-Methylindole is acylated regioselectively at the methyl group when treated with a variety of acyl chlorides in 1,2-dichloroethane in the presence of AlCl3, affording a mild and direct method for the synthesis of 3-(2-oxoalkyl)indoles. The product formation in this one-pot reaction largel ...
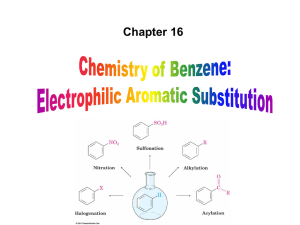
Ex: -F, -Cl, -Br
... Directing effects of substituents on further substitution 1) Activating groups direct further substitution to ortho and para positions Activating groups form resonance structures with increased e- density at ortho and para positions, encouraging electrophiles to attack at these positions. Reson ...
... Directing effects of substituents on further substitution 1) Activating groups direct further substitution to ortho and para positions Activating groups form resonance structures with increased e- density at ortho and para positions, encouraging electrophiles to attack at these positions. Reson ...
Chem 30CL-Lecture 12.. - UCLA Chemistry and Biochemistry
... Some functional groups react with the reagent because they contain electrophilic atoms: -CHO, -COR, -CONR2, -COOR, -C≡N, -NO2, -SO2R, epoxides (ring opening) If more than one of these groups is present, groups that are ...
... Some functional groups react with the reagent because they contain electrophilic atoms: -CHO, -COR, -CONR2, -COOR, -C≡N, -NO2, -SO2R, epoxides (ring opening) If more than one of these groups is present, groups that are ...
Alcohols General formula R-OH hydroxyl group Nomenclature
... rapidly as it is formed. The slow rate-determining step is the formation of the carbocation. Tertiary alcohols react more rapidly than secondary which react more rapidly than primary. e.g. CH3 CH2 20% H2SO4 + H2 O H3C C CH3 H3C C OH 90oC CH3 CH3 CH2 Dehydration follows the H3C OH Saytzeff rule - the ...
... rapidly as it is formed. The slow rate-determining step is the formation of the carbocation. Tertiary alcohols react more rapidly than secondary which react more rapidly than primary. e.g. CH3 CH2 20% H2SO4 + H2 O H3C C CH3 H3C C OH 90oC CH3 CH3 CH2 Dehydration follows the H3C OH Saytzeff rule - the ...
Woodward–Hoffmann rules

The Woodward–Hoffmann rules, devised by Robert Burns Woodward and Roald Hoffmann, are a set of rules in organic chemistry predicting the barrier heights of pericyclic reactions based upon conservation of orbital symmetry. The Woodward–Hoffmann rules can be applied to understand electrocyclic reactions, cycloadditions (including cheletropic reactions), sigmatropic reactions, and group transfer reactions. Reactions are classified as allowed if the electronic barrier is low, and forbidden if the barrier is high. Forbidden reactions can still take place but require significantly more energy.The Woodward–Hoffmann rules were first formulated to explain the striking stereospecificity of electrocyclic reactions under thermal and photochemical control. Thermolysis of the substituted cyclobutene trans-1,2,3,4-tetramethylcyclobutene (1) gave only one diastereomer, the (E,E)-3,4-dimethyl-2,4-hexadiene (2) as shown below; the (Z,Z) and the (E,Z) diastereomers were not detected in the reaction. Similarly, thermolysis of cis-1,2,3,4-tetramethylcyclobutene (3) gave only the (E,Z) diastereomer (4).Due to their elegance and simplicity, the Woodward–Hoffmann rules are credited with first exemplifying the power of molecular orbital theory to experimental chemists. Hoffmann was awarded the 1981 Nobel Prize in Chemistry for this work, shared with Kenichi Fukui who developed a similar model using frontier molecular orbital (FMO) theory; because Woodward had died two years before, he was not eligible to win what would have been his second Nobel Prize for Chemistry.