homogeneous catalysis
... criteria. We have tried to include most of the homogeneous catalytic reactions with proven industrial applications and well-established mechanisms. The basic aim has been to highlight the connections that exist between imaginative academic research and successful technology. In the process, topics a ...
... criteria. We have tried to include most of the homogeneous catalytic reactions with proven industrial applications and well-established mechanisms. The basic aim has been to highlight the connections that exist between imaginative academic research and successful technology. In the process, topics a ...
Title Several Reactions of Isocyanide and Related Compounds
... that (i) copper compounds are excellent catalysts for the carbonylation of aliphatic amines to produce the corresponding formamides; (ii) the catalytic activities of copper compounds are enhanced by the addition of water; and (iii) chloroauric acid catalyzes the carbonylation of aromatic amine where ...
... that (i) copper compounds are excellent catalysts for the carbonylation of aliphatic amines to produce the corresponding formamides; (ii) the catalytic activities of copper compounds are enhanced by the addition of water; and (iii) chloroauric acid catalyzes the carbonylation of aromatic amine where ...
- Sacramento - California State University
... Vanadium has been used as a catalyst for polymerization1, oxidation of alcohols2, sulfides3, and more importantly, allylic alcohols. Epoxides are useful building blocks for natural product synthesis and medicinal chemistry because new functional groups can easily be introduced by nucleophilic additi ...
... Vanadium has been used as a catalyst for polymerization1, oxidation of alcohols2, sulfides3, and more importantly, allylic alcohols. Epoxides are useful building blocks for natural product synthesis and medicinal chemistry because new functional groups can easily be introduced by nucleophilic additi ...
CHEMISTRY 211 FINAL EXAM Wed., December 4, 2002 Name
... briefly, but clearly, what type of reaction is taking place in each case and why this would lead to the ...
... briefly, but clearly, what type of reaction is taking place in each case and why this would lead to the ...
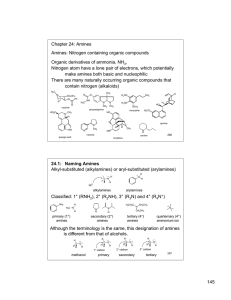
145 Chapter 24: Amines Amines: Nitrogen containing organic
... Symmetrical secondary and tertiary amines are named by adding the prefix di- or tri- to the alkyl group Unsymmetrical secondary and tertiary amines are named as N-substituted primary amines. The largest alkyl group is the parent name, and other alkyl groups are considered N-substituents. H H3CH2C ...
... Symmetrical secondary and tertiary amines are named by adding the prefix di- or tri- to the alkyl group Unsymmetrical secondary and tertiary amines are named as N-substituted primary amines. The largest alkyl group is the parent name, and other alkyl groups are considered N-substituents. H H3CH2C ...
Full-Text PDF
... presence of two ortho positions. The result in entry 7 reinforces the regioselectivity shown in entry 1. A special case of discussion is entry 10 vs. 7. In the first case, where the ortho and para positions were flanked by two substituents, the ortho one was favored. This observation indicates that ...
... presence of two ortho positions. The result in entry 7 reinforces the regioselectivity shown in entry 1. A special case of discussion is entry 10 vs. 7. In the first case, where the ortho and para positions were flanked by two substituents, the ortho one was favored. This observation indicates that ...
Improved Synthesis, Separation, Transition Metal Coordination and
... This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. ...
... This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. ...
Amines
... to -CH2Amides can be reduced by LiAlH4 but NOT the less reactive NaBH4 Typical reagents : LiAlH4 / ether solvent, followed by aqueous work-up. Note that this reaction is different to that of other C=O compounds which reduce to alcohols (for example esters) The nature of the amine obtained depends on ...
... to -CH2Amides can be reduced by LiAlH4 but NOT the less reactive NaBH4 Typical reagents : LiAlH4 / ether solvent, followed by aqueous work-up. Note that this reaction is different to that of other C=O compounds which reduce to alcohols (for example esters) The nature of the amine obtained depends on ...
Alcohols, Phenols , Phenols and Ethers Alcohols
... are higher in comparison to other classes of compounds, namely hydrocarbons, ethers, haloalkanes and haloarenes of comparable molecular masses. For example, ethanol and propane have comparable molecular masses but their boiling points differ widely. The boiling point of methoxymethane is intermediat ...
... are higher in comparison to other classes of compounds, namely hydrocarbons, ethers, haloalkanes and haloarenes of comparable molecular masses. For example, ethanol and propane have comparable molecular masses but their boiling points differ widely. The boiling point of methoxymethane is intermediat ...
HMDS+TMCS+Pyridine - Sigma
... increase in product peak(s) is observed. Derivatization times vary widely, depending upon the specific compound(s) being derivatized. Many compounds are completely derivatized as soon as they dissolve in the reagent. Compounds with poor solubility may require warming. A few compounds will require he ...
... increase in product peak(s) is observed. Derivatization times vary widely, depending upon the specific compound(s) being derivatized. Many compounds are completely derivatized as soon as they dissolve in the reagent. Compounds with poor solubility may require warming. A few compounds will require he ...
PDF - Nanyang Technological University
... a cooperative catalytic system to develop the enantioselective reaction between 1 a and 2 a. The reaction catalyzed by (S)proline/CuBr2 in DMF gave the desired product in 63 % yield and in a 1:1 diastereomeric ratio (d.r.), with virtually no enantioselectivity (4 %/3 % ee) (Table 1, entry 4). The re ...
... a cooperative catalytic system to develop the enantioselective reaction between 1 a and 2 a. The reaction catalyzed by (S)proline/CuBr2 in DMF gave the desired product in 63 % yield and in a 1:1 diastereomeric ratio (d.r.), with virtually no enantioselectivity (4 %/3 % ee) (Table 1, entry 4). The re ...
12_chemistry_impq_CH10_haloalkanes_and_haloarenes_02
... Q4. Of benzene and phenol, which is more easily nitrated and why? Ans. Nitration is an electrophilic substitution. The –OH group in phenol increases the electron density at ortho and para position as follows Since phenol has higher electron density due to electron releasing nature of -OH group , com ...
... Q4. Of benzene and phenol, which is more easily nitrated and why? Ans. Nitration is an electrophilic substitution. The –OH group in phenol increases the electron density at ortho and para position as follows Since phenol has higher electron density due to electron releasing nature of -OH group , com ...
Grignard Reagents brochure
... The reaction of Grignard reagents with carbon dioxide leads to carboxylic acids in moderate to good yields and is one of the most common methods for the synthesis of organic acids. The addition of Grignard reagents to other double bonds like C=S (i.e. in thioesters) and C=N (i.e. nitriles, imines or ...
... The reaction of Grignard reagents with carbon dioxide leads to carboxylic acids in moderate to good yields and is one of the most common methods for the synthesis of organic acids. The addition of Grignard reagents to other double bonds like C=S (i.e. in thioesters) and C=N (i.e. nitriles, imines or ...
HOMOLOGATION OF HETEROCYCLES BY A SEQUENTIAL REDUCTIVE OPENING LITHIATION – S
... The introduction of a carbonyl moiety into an organic molecule using carbon monoxide requires the presence of transition metal complex functioning as a catalyst or as a stoichiometric reactant. The insertion of carbon monoxide into a carbon-heteroatom bond of a heterocyclic compound comprises a simu ...
... The introduction of a carbonyl moiety into an organic molecule using carbon monoxide requires the presence of transition metal complex functioning as a catalyst or as a stoichiometric reactant. The insertion of carbon monoxide into a carbon-heteroatom bond of a heterocyclic compound comprises a simu ...
Isoindolone Formation via Intramolecular Diels
... A number of different approaches were attempted, but only one realistic alternative was found, utilising an intramolecular Diels−Alder approach1 to build the isoindolone ring (Scheme 4). The initial Vilsmeier reaction,2 whilst operationally straightforward, did present significant challenges due to th ...
... A number of different approaches were attempted, but only one realistic alternative was found, utilising an intramolecular Diels−Alder approach1 to build the isoindolone ring (Scheme 4). The initial Vilsmeier reaction,2 whilst operationally straightforward, did present significant challenges due to th ...
Woodward–Hoffmann rules

The Woodward–Hoffmann rules, devised by Robert Burns Woodward and Roald Hoffmann, are a set of rules in organic chemistry predicting the barrier heights of pericyclic reactions based upon conservation of orbital symmetry. The Woodward–Hoffmann rules can be applied to understand electrocyclic reactions, cycloadditions (including cheletropic reactions), sigmatropic reactions, and group transfer reactions. Reactions are classified as allowed if the electronic barrier is low, and forbidden if the barrier is high. Forbidden reactions can still take place but require significantly more energy.The Woodward–Hoffmann rules were first formulated to explain the striking stereospecificity of electrocyclic reactions under thermal and photochemical control. Thermolysis of the substituted cyclobutene trans-1,2,3,4-tetramethylcyclobutene (1) gave only one diastereomer, the (E,E)-3,4-dimethyl-2,4-hexadiene (2) as shown below; the (Z,Z) and the (E,Z) diastereomers were not detected in the reaction. Similarly, thermolysis of cis-1,2,3,4-tetramethylcyclobutene (3) gave only the (E,Z) diastereomer (4).Due to their elegance and simplicity, the Woodward–Hoffmann rules are credited with first exemplifying the power of molecular orbital theory to experimental chemists. Hoffmann was awarded the 1981 Nobel Prize in Chemistry for this work, shared with Kenichi Fukui who developed a similar model using frontier molecular orbital (FMO) theory; because Woodward had died two years before, he was not eligible to win what would have been his second Nobel Prize for Chemistry.