ert207 analytical chemistry
... What is Analytical Chemistry? • Concerned with the chemical characterization of matter and the answer of : 1) What is it (Qualitative) –identification of elements, ions or compound 2) How much is it (Quantitative) ...
... What is Analytical Chemistry? • Concerned with the chemical characterization of matter and the answer of : 1) What is it (Qualitative) –identification of elements, ions or compound 2) How much is it (Quantitative) ...
Ch. 1 Introduction: Matter and Measurement
... properties of atoms and molecules. The understanding of matter at the molecular level gives us the ability to control that matter. First semester General Chemistry – the primary goal is to learn the fundamental principles of Chemistry. ...
... properties of atoms and molecules. The understanding of matter at the molecular level gives us the ability to control that matter. First semester General Chemistry – the primary goal is to learn the fundamental principles of Chemistry. ...
Mineral Web Quest
... 3. What eight elements make up over 98% of the Earth's crust? List in order from largest percentage to smallest. ...
... 3. What eight elements make up over 98% of the Earth's crust? List in order from largest percentage to smallest. ...
Chemistry Study Guide
... Conservation of Mass- Mass cannot be created or destroyed in a chemical reaction. ...
... Conservation of Mass- Mass cannot be created or destroyed in a chemical reaction. ...
Chemistry Study Guide
... Conservation of Mass- Mass cannot be created or destroyed in a chemical reaction. ...
... Conservation of Mass- Mass cannot be created or destroyed in a chemical reaction. ...
Name Objective 1: Matter and Energy C3H8 + 5O2 → 3CO2 + 4H2O
... 16. Which two compounds contain the same total number of atoms? (8.5D) a. C3H8 and C2H6 b. NO2 and KCl c. 2Li2S and Be4Cl2 d. 2CO and CO2 17. All of the following are indicators of a chemical change except — (8.5E) a. formation of a gas b. change in temperature c. change in the state of matter d. fo ...
... 16. Which two compounds contain the same total number of atoms? (8.5D) a. C3H8 and C2H6 b. NO2 and KCl c. 2Li2S and Be4Cl2 d. 2CO and CO2 17. All of the following are indicators of a chemical change except — (8.5E) a. formation of a gas b. change in temperature c. change in the state of matter d. fo ...
The structure of Matter
... O Compounds that contain only carbon and hydrogen are called hydrocarbons. O Two of the simplest hydrocarbons are methane and ethane. O Many hydrocarbons are used as fuels. ...
... O Compounds that contain only carbon and hydrogen are called hydrocarbons. O Two of the simplest hydrocarbons are methane and ethane. O Many hydrocarbons are used as fuels. ...
PowerPoint Template
... the total mass of substances does not change during a chemical reaction - Antoine Lavoisier (1 743-1 794) The number of substances may change, but the total amount of matter remains constant. ...
... the total mass of substances does not change during a chemical reaction - Antoine Lavoisier (1 743-1 794) The number of substances may change, but the total amount of matter remains constant. ...
•What makes up an atom? Draw an atom
... WE ARE MADE OF ELEMENTS • Element - Pure substance that can’t be broken into other types of matter • Each element has its own symbol and specific number of protons ...
... WE ARE MADE OF ELEMENTS • Element - Pure substance that can’t be broken into other types of matter • Each element has its own symbol and specific number of protons ...
Chapter 3 Note Packet
... ____________is a separation technique for homogenous mixtures that results in the formation of pure solid particles from a solution containing the dissolved substance. Mixtures of Matter ...
... ____________is a separation technique for homogenous mixtures that results in the formation of pure solid particles from a solution containing the dissolved substance. Mixtures of Matter ...
States of Matter
... Classify changes of state in terms of endothermic and exothermic processes Classify mixtures as being homogenous or heterogeneous Distinguish among elements, atoms, compounds, and mixtures Distinguish between a chemical and physical change. Demonstrate the conservation of energy in calculations usin ...
... Classify changes of state in terms of endothermic and exothermic processes Classify mixtures as being homogenous or heterogeneous Distinguish among elements, atoms, compounds, and mixtures Distinguish between a chemical and physical change. Demonstrate the conservation of energy in calculations usin ...
Structure-activity relationships
... and England, established cooperative relationships with academic labs. Postulated by Paul Ehrlich in 1906 following more than a decade of research, the concept that synthetic chemicals could selectively kill or immobilize parasites, bacteria, and other invasive disease-causing microbes would eventua ...
... and England, established cooperative relationships with academic labs. Postulated by Paul Ehrlich in 1906 following more than a decade of research, the concept that synthetic chemicals could selectively kill or immobilize parasites, bacteria, and other invasive disease-causing microbes would eventua ...
Intro to Chem
... A chemical reaction (rxn) has two parts the reactants and the products. Reactant – substance present at the start of the rxn Product – substance produced in the rxn. H2 + O2 → 2H2O ...
... A chemical reaction (rxn) has two parts the reactants and the products. Reactant – substance present at the start of the rxn Product – substance produced in the rxn. H2 + O2 → 2H2O ...
Valence Electrons and Chemical Bonding
... eight electrons in their outer energy level or, in the case of elements 1-5, two in their outer shell level. ...
... eight electrons in their outer energy level or, in the case of elements 1-5, two in their outer shell level. ...
Chem Bonding Notes
... 1. At STP, the element oxygen can exist as either O 2 or O 3 gas molecules. These two forms of the element have (1) the same chemical and physical properties (2) the same chemical properties and different physical properties (3) different chemical properties and the same physical properties (4) diff ...
... 1. At STP, the element oxygen can exist as either O 2 or O 3 gas molecules. These two forms of the element have (1) the same chemical and physical properties (2) the same chemical properties and different physical properties (3) different chemical properties and the same physical properties (4) diff ...
Chemistry Semester One Exam Review Name:
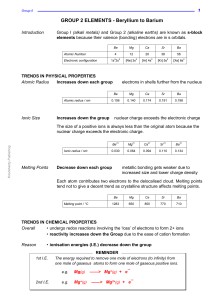
... 11. Write the electron configurations for the following elements. LithiumNitrogenZincBromineBarium12. What is the characteristic set of valence electrons for the following groups on the periodic table? Alkali metals (1); alkaline earth metals (2); halogens (17); noble gases (18) ...
... 11. Write the electron configurations for the following elements. LithiumNitrogenZincBromineBarium12. What is the characteristic set of valence electrons for the following groups on the periodic table? Alkali metals (1); alkaline earth metals (2); halogens (17); noble gases (18) ...
What is a Mineral?
... Questions to ask yourself when determining if an item is a mineral or not: Is it non-living material? Is it a solid? Is it formed in nature? Does it have a crystalline structure? ...
... Questions to ask yourself when determining if an item is a mineral or not: Is it non-living material? Is it a solid? Is it formed in nature? Does it have a crystalline structure? ...
Headline Text 28 Point Color Text 2
... How molecular motors work Computer modeling in support of chemical and drug design • Polymer delivery systems • Catalysts • Small molecule drugs ...
... How molecular motors work Computer modeling in support of chemical and drug design • Polymer delivery systems • Catalysts • Small molecule drugs ...
Getting to Rational Drug Design — at Last
... that is unfolding in the pharmaceutical industry. This shift is being driven by the challenges posed by the many new structures developed through combinatorial chemistry, ever-increasing research and development costs, clinical trials that continue to grow in complexity and costreduction pressures f ...
... that is unfolding in the pharmaceutical industry. This shift is being driven by the challenges posed by the many new structures developed through combinatorial chemistry, ever-increasing research and development costs, clinical trials that continue to grow in complexity and costreduction pressures f ...
Classification of Matter
... • An element is a pure substance that is made up of only one kind of atom. What are atoms? • Atoms are the smallest unit of an element that maintains the identity of that element. What are compounds? • A compound is a substance made up of two or more elements that are chemically bonded. ...
... • An element is a pure substance that is made up of only one kind of atom. What are atoms? • Atoms are the smallest unit of an element that maintains the identity of that element. What are compounds? • A compound is a substance made up of two or more elements that are chemically bonded. ...