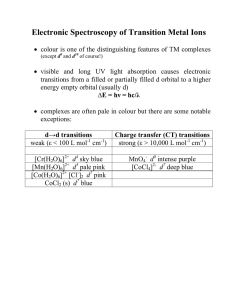
QuantumChem - II
... – Replace the core electrons with a single function that represents reasonably accurately (but much more efficiently) the combined nuclear-electronic core to the remaining electrons – Effective Core Potential (ECP), or “pseudopotential” – Relativistic effects can be folded into the ECP ...
... – Replace the core electrons with a single function that represents reasonably accurately (but much more efficiently) the combined nuclear-electronic core to the remaining electrons – Effective Core Potential (ECP), or “pseudopotential” – Relativistic effects can be folded into the ECP ...
Raman spectroscopy: Watching a molecule breathe
... noise scales with 1/(N∙V), where N is the number of molecules and V is the number of vibrational states excited. Thus, knowing the number of vibrational states involved, they showed by statistical analysis that it is possible to distinguish whether one addresses a single molecule or multiple molecul ...
... noise scales with 1/(N∙V), where N is the number of molecules and V is the number of vibrational states excited. Thus, knowing the number of vibrational states involved, they showed by statistical analysis that it is possible to distinguish whether one addresses a single molecule or multiple molecul ...
The Modern Atomic Model
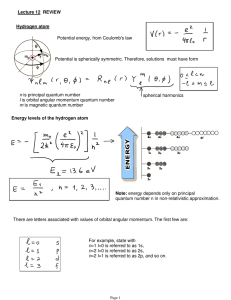
... Bohr Model of the Atom (review) •Energy levels contain electrons. •Electrons travel around the nucleus. •Different orbitals varied by different quantum (energy). •Gaps between energy levels were not equal. ...
... Bohr Model of the Atom (review) •Energy levels contain electrons. •Electrons travel around the nucleus. •Different orbitals varied by different quantum (energy). •Gaps between energy levels were not equal. ...
Answers to questions on test #2
... or “The wavefunction of a many-electron system must be antisymmetric with respect to interchange of the coordinates of any two of its electrons.” or “In an atom, no two electron can have the same four quantum numbers” (strictly speaking, the last statement is a consequence of the Pauli principle, it ...
... or “The wavefunction of a many-electron system must be antisymmetric with respect to interchange of the coordinates of any two of its electrons.” or “In an atom, no two electron can have the same four quantum numbers” (strictly speaking, the last statement is a consequence of the Pauli principle, it ...
quantum mechanical model
... then yellow, then white when heated to higher and higher temperatures? ...
... then yellow, then white when heated to higher and higher temperatures? ...
Nonradiative electron and energy transfer. Explicit
... functions are exact up to second-order in the system–bath coupling, their analytical forms explicitly consist of a weighted sum of delta functions in the frequency domain. Practically, on the other hand, they are alternatively replaced by phenomenological expressions, like Debye’s, Ohmic spectral fu ...
... functions are exact up to second-order in the system–bath coupling, their analytical forms explicitly consist of a weighted sum of delta functions in the frequency domain. Practically, on the other hand, they are alternatively replaced by phenomenological expressions, like Debye’s, Ohmic spectral fu ...
Solutions to the exam itself are now available.
... (a) What is the electron configuration of the Li atom produced when ground state Li absorbs this photon? Just as in the Na atom you studied in lab and we discussed in class, the longest wavelength excitation (smallest energy excitation) promotes the s valence electron to the p orbital of the same pr ...
... (a) What is the electron configuration of the Li atom produced when ground state Li absorbs this photon? Just as in the Na atom you studied in lab and we discussed in class, the longest wavelength excitation (smallest energy excitation) promotes the s valence electron to the p orbital of the same pr ...
Study Guide: Chapter 4 - the Arrangement of Electrons in Atoms
... Study Guide: Chapter 4 - the Arrangement of Electrons in Atoms 1. Understand the relationship between a light wave’s frequency and wavelength; Know how to calculate wavelength given frequency and frequency given wavelength (MEMORIZE FORMULA) – work a few practice problems 2. Understand the relations ...
... Study Guide: Chapter 4 - the Arrangement of Electrons in Atoms 1. Understand the relationship between a light wave’s frequency and wavelength; Know how to calculate wavelength given frequency and frequency given wavelength (MEMORIZE FORMULA) – work a few practice problems 2. Understand the relations ...
Chapter 9d Introduction to Quantum Mechanics
... very large, the energy approaches zero. From above we can see that from n=6 to n = infinity, all the corresponding energy levels are packed between – 0.38eV and zero. Therefore, at the very large region of n, the discrete energy levels can be recognized as continuous! ...
... very large, the energy approaches zero. From above we can see that from n=6 to n = infinity, all the corresponding energy levels are packed between – 0.38eV and zero. Therefore, at the very large region of n, the discrete energy levels can be recognized as continuous! ...
Franck–Condon principle
The Franck–Condon principle is a rule in spectroscopy and quantum chemistry that explains the intensity of vibronic transitions. Vibronic transitions are the simultaneous changes in electronic and vibrational energy levels of a molecule due to the absorption or emission of a photon of the appropriate energy. The principle states that during an electronic transition, a change from one vibrational energy level to another will be more likely to happen if the two vibrational wave functions overlap more significantly.