* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Uncertainty Principle
EPR paradox wikipedia , lookup
Atomic orbital wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Franck–Condon principle wikipedia , lookup
Tight binding wikipedia , lookup
Noether's theorem wikipedia , lookup
Coherent states wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
Renormalization wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Wheeler's delayed choice experiment wikipedia , lookup
Atomic theory wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Delayed choice quantum eraser wikipedia , lookup
Hydrogen atom wikipedia , lookup
Renormalization group wikipedia , lookup
Particle in a box wikipedia , lookup
Electron scattering wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Wave–particle duality wikipedia , lookup
Matter wave wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
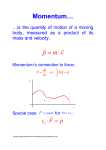
Uncertainty Principle Observation Observations generally require energy interacting with matter. • • • • • Light on a ruler Radar on a car Echoes in a canyon Touch on a surface Voltmeter in a circuit In many cases this is scattering of EM waves. Impulse p Ft Direct contact creates an impulse. • Change in momentum EM waves have momentum as photons. p h reflected photon incident photon Moving charge • Momentum transfer by reflection • Planck’s law Atomic Scale At the atomic scale the momentum of a photon may be comparable to the momentum of a particle. The photon as a wave can p only be measured in position to about one wavelength. h reflected photon If momentum is transferred the target has a momentum uncertainty. x incident photon Moving charge Uncertainty The product of the uncertainties in position and momentum is a limit on measurement. • Heisenberg Uncertainty Principle The relationship is based on the angular frequency. • Shift by a factor of 2p • Use constant h-bar h = h/2p x p 2 Stop Motion p 2 x x 2 p The uncertainty principle says that if the position is perfectly known the momentum is unknown. If the momentum is perfectly known then the position is unknown. The two variables are interrelated. • Conjugate variables Harold Edgerton (1964) Freezing Time The energy of a wave is related to its frequency. Energy and frequency complement like momentum and wavelength. The uncertainty principle applies to energy and time as well. E hf E p h f E t 2 Confined Space An atomic nucleus is 10-14 m in diameter. Find the total energy in eV of an electron confined to that space. Use hc = 1240 eV nm c 197 eV nm • x = The uncertainty principle matches distance to momentum. • Energy units here c pc 9.85 MeV 2x Apply relativity to get total 10-5 nm energy. • Rest mass relatively small Relativity may matter. • mc2 = 0.511 MeV E (mc2 ) 2 ( pc) 2 9.86 MeV Indeterminate Newtonian physics is viewed as a deterministic system. • Initial positions allow calculation of final states • Knowledge of all past variables implies future knowledge Quantum physics has an indeterminate element. • Conjugate variables are of limited measurability • Impossible to have precise initial state • Cannot know precise future states next