Alcohols, Phenols and Ethers
... The reaction with R’COOH and (R’ CO)2O is reversible, so cone, H2SO4 is used to remove water. The reaction with R’ COCI is carried out in the presence of pyridine so as to neutralise HCI which is formed during the reaction. ...
... The reaction with R’COOH and (R’ CO)2O is reversible, so cone, H2SO4 is used to remove water. The reaction with R’ COCI is carried out in the presence of pyridine so as to neutralise HCI which is formed during the reaction. ...
File - Mr Weng`s IB Chemistry
... • Writing equations for the complete combustion of alcohols. • Writing equations for the oxidation reactions of primary and secondary alcohols (using acidified potassium dichromate(VI) or potassium manganate(VII) as oxidizing agents). Explanation of distillation and reflux in the isolation of the al ...
... • Writing equations for the complete combustion of alcohols. • Writing equations for the oxidation reactions of primary and secondary alcohols (using acidified potassium dichromate(VI) or potassium manganate(VII) as oxidizing agents). Explanation of distillation and reflux in the isolation of the al ...
Biosynthesis of Amino Acids - Tennessee Wesleyan College
... 2) Duplicate the Amino Acid Synthesis Overview Flow Chart. Expand the sections need to show the synthesis of Glutamate, Glutamine, Proline, Arginine, Serine, Cysteine, Glycine, and starting from Chorismate Tryptophan, Tyrosine and Phenylalanine. (40 points!!) ...
... 2) Duplicate the Amino Acid Synthesis Overview Flow Chart. Expand the sections need to show the synthesis of Glutamate, Glutamine, Proline, Arginine, Serine, Cysteine, Glycine, and starting from Chorismate Tryptophan, Tyrosine and Phenylalanine. (40 points!!) ...
Year 1 Foundation course, section B2
... Aromatic compounds are stable cyclic systems of conjugated double bonds. There must be 2n+1 double bonds for the system to be stable. You can illustrate them in two ways, the first of which is more accurate. The structure consists of a system of six sigma (s) bonds with a p bond system on top which ...
... Aromatic compounds are stable cyclic systems of conjugated double bonds. There must be 2n+1 double bonds for the system to be stable. You can illustrate them in two ways, the first of which is more accurate. The structure consists of a system of six sigma (s) bonds with a p bond system on top which ...
14. The Direct and Enantioselective Organocatalytic -Oxidation of Aldehydes
... presence of various BINAP-AgX catalysts.5 On the basis of these studies, as well as the recent elegant work by List,6 we were prompted to consider the direct, proline-catalyzed R-oxyamination of aldehydes with nitrosobenzene (eq 2).7 While Yamamoto has shown that uncatalyzed reactions of silyl keten ...
... presence of various BINAP-AgX catalysts.5 On the basis of these studies, as well as the recent elegant work by List,6 we were prompted to consider the direct, proline-catalyzed R-oxyamination of aldehydes with nitrosobenzene (eq 2).7 While Yamamoto has shown that uncatalyzed reactions of silyl keten ...
Problem 14. MAGNESIUM DETERMINATION
... The superposition principle is applicable to quantum systems only and is not valid when applied to macrosystems. To illustrate this idea, E. Schrödinger proposed the following mental experiment. Consider the Geiger counter which detects the entering electrons. The counter is connected to a device wh ...
... The superposition principle is applicable to quantum systems only and is not valid when applied to macrosystems. To illustrate this idea, E. Schrödinger proposed the following mental experiment. Consider the Geiger counter which detects the entering electrons. The counter is connected to a device wh ...
polymerisation
... Recall and understand the different types of structures of poly(propene) Recall the properties of some common polymers Recall that polyesters and polyamides are formed by condensation polymerisation Recall the properties and uses of some condensation polymers Work out the repeating unit in condensat ...
... Recall and understand the different types of structures of poly(propene) Recall the properties of some common polymers Recall that polyesters and polyamides are formed by condensation polymerisation Recall the properties and uses of some condensation polymers Work out the repeating unit in condensat ...
No Slide Title
... Recall and understand the different types of structures of poly(propene) Recall the properties of some common polymers Recall that polyesters and polyamides are formed by condensation polymerisation Recall the properties and uses of some condensation polymers Work out the repeating unit in condensat ...
... Recall and understand the different types of structures of poly(propene) Recall the properties of some common polymers Recall that polyesters and polyamides are formed by condensation polymerisation Recall the properties and uses of some condensation polymers Work out the repeating unit in condensat ...
1-1 EXPERIMENT 1: Preparation and Reactivity of Alkyl Halides
... one-step, concerted, SN2 reaction. Therefore, the reaction occurs most quickly when attack at the carbon that bears the halogen (X) is least hindered sterically. For alkyl halides, the order of reactivity is primary > secondary > tertiary. (ii) Lucas Test: Test for SN1 The reactivity of the product ...
... one-step, concerted, SN2 reaction. Therefore, the reaction occurs most quickly when attack at the carbon that bears the halogen (X) is least hindered sterically. For alkyl halides, the order of reactivity is primary > secondary > tertiary. (ii) Lucas Test: Test for SN1 The reactivity of the product ...
unit c3 – chemistry in action checklist
... Demonstrate an understanding of the consequential effects of these changes on the rate of attainment of equilibrium and of the need to use a catalyst Describe how, in industrial reactions such as the Haber process, the temperature, pressure and catalyst used produce an acceptable yield in an accepta ...
... Demonstrate an understanding of the consequential effects of these changes on the rate of attainment of equilibrium and of the need to use a catalyst Describe how, in industrial reactions such as the Haber process, the temperature, pressure and catalyst used produce an acceptable yield in an accepta ...
A Convenient Preparation of Volatile Acid Chlorides
... rides for the preparation of other acid chlorides distilled through a small column directly out of the has been neglected for the most part. Adams and reaction mixture. The reaction is general, limUlichl have reported that they could obtain al- ited only by the volatility of the acid chloridemost qu ...
... rides for the preparation of other acid chlorides distilled through a small column directly out of the has been neglected for the most part. Adams and reaction mixture. The reaction is general, limUlichl have reported that they could obtain al- ited only by the volatility of the acid chloridemost qu ...
Reaction of orthoesters with alcohols in the presence of acidic
... alcohols, the acetylated product could be isolated in only trace amounts. As the relative abundance of these products mainly depends upon the nature of the catalyst used, the product formation can be correlated to the degree of stabilization of the positively charged intermediate on the solid acids. ...
... alcohols, the acetylated product could be isolated in only trace amounts. As the relative abundance of these products mainly depends upon the nature of the catalyst used, the product formation can be correlated to the degree of stabilization of the positively charged intermediate on the solid acids. ...
Proceedings of the Indiana Academy of Science
... hydrocarbons (saturated, unsaturated, or aromatic), ketones, and phenols. The inclusion of a larger number of functional groups introduces more organic reactions and affords a broader view of the subject. Salts of amines and carboxylic acids are excluded as possibilities in the identification proced ...
... hydrocarbons (saturated, unsaturated, or aromatic), ketones, and phenols. The inclusion of a larger number of functional groups introduces more organic reactions and affords a broader view of the subject. Salts of amines and carboxylic acids are excluded as possibilities in the identification proced ...
LABORATORY 5 DETECTION OF FUNCTIONAL GROUPS IN
... 5. Reaction of phenol with ferric chloride Ferric chloride reacts with the hydroxyl groups of phenols and phenol derivatives and forms stable-colored complexes: violet, violet-bluish, blue, green and red. This reaction depends on the structure and presence of complex-created groups (aldehyde, ketone ...
... 5. Reaction of phenol with ferric chloride Ferric chloride reacts with the hydroxyl groups of phenols and phenol derivatives and forms stable-colored complexes: violet, violet-bluish, blue, green and red. This reaction depends on the structure and presence of complex-created groups (aldehyde, ketone ...
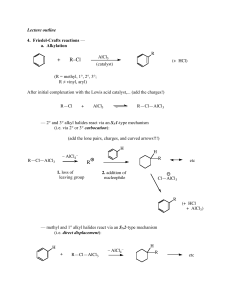
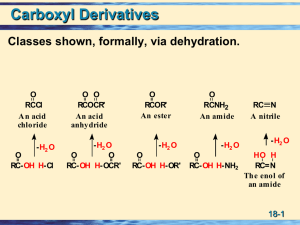
Acid derivatives
... by elimination. Acid catalysts act to increase the electrophilicity of the acyl reactant; whereas, base catalysts act on the nucleophilic reactant to increase its reactivity. In principle all steps are reversible, but in practice many reactions of this kind are irreversible unless changes in the rea ...
... by elimination. Acid catalysts act to increase the electrophilicity of the acyl reactant; whereas, base catalysts act on the nucleophilic reactant to increase its reactivity. In principle all steps are reversible, but in practice many reactions of this kind are irreversible unless changes in the rea ...
A Straightforward Route to Enantiopure Pyrrolizidines and
... Since in a similar case [24] even treatment with excess trifluoroperacetic acid (CH2Cl2, reflux) and other reagents [27] was unsuccessful, we tested potassium permanganate as a strong oxidant. In a first experiment, sulfinate 16 was reacted with two mole equivalents of KMnO4 in a pyridinewater mixtu ...
... Since in a similar case [24] even treatment with excess trifluoroperacetic acid (CH2Cl2, reflux) and other reagents [27] was unsuccessful, we tested potassium permanganate as a strong oxidant. In a first experiment, sulfinate 16 was reacted with two mole equivalents of KMnO4 in a pyridinewater mixtu ...
Zn mediated regioselective Barbier reaction of propargylic bromides
... regioselective synthesis of allenic alcohols in media containing water is not documented. Here we describe the regiospecific Barbier reaction of two representative model compounds giving exclusively the corresponding substituted propargylic or allenic alcohol. Results and Discussion In our investiga ...
... regioselective synthesis of allenic alcohols in media containing water is not documented. Here we describe the regiospecific Barbier reaction of two representative model compounds giving exclusively the corresponding substituted propargylic or allenic alcohol. Results and Discussion In our investiga ...
Chapter 23 Functional Groups
... of the previous classes of organic compounds are related by oxidation and reduction reactions What is oxidation-reduction? –Oxidation: the gain of oxygen, loss of hydrogen, or loss of e-1 –Reduction: the loss of oxygen, gain of hydrogen, or gain of e-1 ...
... of the previous classes of organic compounds are related by oxidation and reduction reactions What is oxidation-reduction? –Oxidation: the gain of oxygen, loss of hydrogen, or loss of e-1 –Reduction: the loss of oxygen, gain of hydrogen, or gain of e-1 ...
PREPARATORY PROBLEMS (Theoretical)
... The natural tendency of any chemical reaction to proceed in a certain direction at constant temperature and pressure is determined by the sign of the Gibbs energy of the reaction, DG. This is the universal principle. If DG < 0, the reaction can proceed predominantly in the forward direction (a produ ...
... The natural tendency of any chemical reaction to proceed in a certain direction at constant temperature and pressure is determined by the sign of the Gibbs energy of the reaction, DG. This is the universal principle. If DG < 0, the reaction can proceed predominantly in the forward direction (a produ ...
PREPARATORY PROBLEMS (Theoretical)
... The natural tendency of any chemical reaction to proceed in a certain direction at constant temperature and pressure is determined by the sign of the Gibbs energy of the reaction, DG. This is the universal principle. If DG < 0, the reaction can proceed predominantly in the forward direction (a produ ...
... The natural tendency of any chemical reaction to proceed in a certain direction at constant temperature and pressure is determined by the sign of the Gibbs energy of the reaction, DG. This is the universal principle. If DG < 0, the reaction can proceed predominantly in the forward direction (a produ ...
Petasis reaction

The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the chemical reaction of an amine, aldehyde, and vinyl- or aryl-boronic acid to form substituted amines.Reported in 1993 by Nicos Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine, the Petasis reaction can be described as a variation of the Mannich reaction. Rather than generating an enolate to form the substituted amine product, in the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry and drug discovery.