Carbonyl Compounds_ Properties and Reactions
... Weaker polarity means aldehydes and ketones mix well with polar solvents such as water and will dissolve many organic compounds. ...
... Weaker polarity means aldehydes and ketones mix well with polar solvents such as water and will dissolve many organic compounds. ...
ch15[1].
... to -OR or -OAr. • Name the alkyl or aryl group bonded to oxygen followed by the name of the acid. • Change the suffix -ic acid to -ate. O O ...
... to -OR or -OAr. • Name the alkyl or aryl group bonded to oxygen followed by the name of the acid. • Change the suffix -ic acid to -ate. O O ...
Chapter 18
... Upon application of light, a cis/trans interconversion occurs which is converted into an electrochemical impulse by affecting the concentration of Ca2+ crossing a cell membrane ...
... Upon application of light, a cis/trans interconversion occurs which is converted into an electrochemical impulse by affecting the concentration of Ca2+ crossing a cell membrane ...
Chemistry
... Compounds of nitrogen: ammonia – manufacture by Haber’s process, properties – basic character, reaction with ZnSO4 and Cu2+ ion. Nitric acid – manufacture by Ostwald’s process , laboratory method – from NaNO3, properties – oxidizing properties – dilute HNO3 with Zn and Cu, concentrated HNO3 with Cu, ...
... Compounds of nitrogen: ammonia – manufacture by Haber’s process, properties – basic character, reaction with ZnSO4 and Cu2+ ion. Nitric acid – manufacture by Ostwald’s process , laboratory method – from NaNO3, properties – oxidizing properties – dilute HNO3 with Zn and Cu, concentrated HNO3 with Cu, ...
click - Chemsheets
... • Acidified potassium dichromate, contains Cr2O72• Used to test for alcohols (1y and 2y) & aldehydes – goes from orange Cr2O72- to green Cr3+ • Reduced from Cr(+6) to Cr(+3) ...
... • Acidified potassium dichromate, contains Cr2O72• Used to test for alcohols (1y and 2y) & aldehydes – goes from orange Cr2O72- to green Cr3+ • Reduced from Cr(+6) to Cr(+3) ...
The Hydroxylation of Aromatic Nitro Compounds by Alkalies
... should diminish the yield; but no such diminution occurs. The only remaining product is water; and this is now believed to render the potassium hydroxide incapable of further reaction by coating the surface. Wohl's statement that the hydroxylation proceeds in the absence of air Is true. but then the ...
... should diminish the yield; but no such diminution occurs. The only remaining product is water; and this is now believed to render the potassium hydroxide incapable of further reaction by coating the surface. Wohl's statement that the hydroxylation proceeds in the absence of air Is true. but then the ...
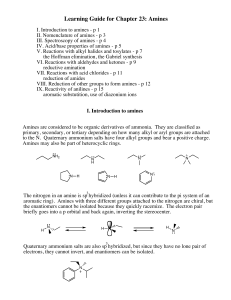
Learning Guide for Chapter 23: Amines
... Once an amine has been protonated, it is an ionic compound. Amine salts are solids, and are usually soluble in water but insoluble in organic solvents. Most biologically active amines are used as their amine salts, which dissolve to make injectable or drinkable water solutions, and are also less pro ...
... Once an amine has been protonated, it is an ionic compound. Amine salts are solids, and are usually soluble in water but insoluble in organic solvents. Most biologically active amines are used as their amine salts, which dissolve to make injectable or drinkable water solutions, and are also less pro ...
Unit D: Quantitative Relationships in Chemical Change
... For each of the following questions, write a balanced chemical equation and then use the mole ratio to answer the question. 1. A student mixes together a solution of silver nitrate with a solution of sodium chromate and a precipitate forms. What amount of precipitate will form if the student has rea ...
... For each of the following questions, write a balanced chemical equation and then use the mole ratio to answer the question. 1. A student mixes together a solution of silver nitrate with a solution of sodium chromate and a precipitate forms. What amount of precipitate will form if the student has rea ...
L22 - Supplementary Student Notes Package
... For each of the following questions, write a balanced chemical equation and then use the mole ratio to answer the question. 1. A student mixes together a solution of silver nitrate with a solution of sodium chromate and a precipitate forms. What amount of precipitate will form if the student has rea ...
... For each of the following questions, write a balanced chemical equation and then use the mole ratio to answer the question. 1. A student mixes together a solution of silver nitrate with a solution of sodium chromate and a precipitate forms. What amount of precipitate will form if the student has rea ...
Fluorine notes- EFFECT OF POLYFLUOROALKYL GROUPS IN
... Methylene groups appear in the field of 2880 – 2980 cm–1 in the form of weak and medium bands, the absorption band of CF2 -H bond lies in the field of higher wave numbers 2995 – 3050 cm–1 because of the presence of electronegative fluorine atoms, very weak intensity is typical for it. The absorption ...
... Methylene groups appear in the field of 2880 – 2980 cm–1 in the form of weak and medium bands, the absorption band of CF2 -H bond lies in the field of higher wave numbers 2995 – 3050 cm–1 because of the presence of electronegative fluorine atoms, very weak intensity is typical for it. The absorption ...
Course Content (Laboratory)
... name compounds containing common functional groups using the IUPAC system of nomenclature; predict major organic products for reactions involving functional groups studied; give simple mechanisms for the following reaction types (SN1, SN2, E1, E2, electrophilic addition and nucleophilic addition); u ...
... name compounds containing common functional groups using the IUPAC system of nomenclature; predict major organic products for reactions involving functional groups studied; give simple mechanisms for the following reaction types (SN1, SN2, E1, E2, electrophilic addition and nucleophilic addition); u ...
OXIDATION - organicchem.org
... 1. Cold, dilute, permanganate (KMnO4) can be used to convert alkenes to cis-diols 2. Osmium tetroxide (toxic, expensive, used in catalytic amount) (OsO4) can be used to convert alkenes to cis diols B. Alkenes can be converted to carbonyl compounds (aldehydes and ketones) through a cis-diol intermedi ...
... 1. Cold, dilute, permanganate (KMnO4) can be used to convert alkenes to cis-diols 2. Osmium tetroxide (toxic, expensive, used in catalytic amount) (OsO4) can be used to convert alkenes to cis diols B. Alkenes can be converted to carbonyl compounds (aldehydes and ketones) through a cis-diol intermedi ...
Chemistry
... 2. Explain the difference between 10, 20 and 30 alkyl halides, with suitable examples. 3. Allyl halides are more reactive than alkyl halides. Why? 4. What are the reagents required prepare alkyl halide from the following (i) alcohol (ii) alkene (iv) alkanes 5. Explain the reactions used to convert b ...
... 2. Explain the difference between 10, 20 and 30 alkyl halides, with suitable examples. 3. Allyl halides are more reactive than alkyl halides. Why? 4. What are the reagents required prepare alkyl halide from the following (i) alcohol (ii) alkene (iv) alkanes 5. Explain the reactions used to convert b ...
(Z)-Tamoxifen and Tetrasubstituted Alkenes and Dienes via a Regio
... Our synthesis commenced with a Sonogashira crosscoupling of the aryl halide 15 with propargyl alcohol 16 to give 17 (83%). This substituted alkynol was then subjected to the standard carbometalation protocol with phenylmagnesium chloride followed by the addition of Pd(PPh3)4 and phenyl iodide as the ...
... Our synthesis commenced with a Sonogashira crosscoupling of the aryl halide 15 with propargyl alcohol 16 to give 17 (83%). This substituted alkynol was then subjected to the standard carbometalation protocol with phenylmagnesium chloride followed by the addition of Pd(PPh3)4 and phenyl iodide as the ...
+ :O
... sodium bicarbonate: Carboxylic acids will dissolve while (water insoluble) phenols will not. Both will dissolve in an aqueous solution of a strong base like NaOH. ...
... sodium bicarbonate: Carboxylic acids will dissolve while (water insoluble) phenols will not. Both will dissolve in an aqueous solution of a strong base like NaOH. ...
Ester-containing polyols having halogen and phosphorus atoms
... the polyesters. Thus, polyester amides may be obtained 30 are generally prepared by heating the reactants at tem peratures between 25° C. and 150° C. preferably between ...
... the polyesters. Thus, polyester amides may be obtained 30 are generally prepared by heating the reactants at tem peratures between 25° C. and 150° C. preferably between ...
8.5 Translation - Science With Ms. Ortiz
... • Regardless of the organism, codons code for the same amino acid. ...
... • Regardless of the organism, codons code for the same amino acid. ...
Carboxylic acids, esters, and other acid derivatives
... For IUPAC naming of this structure, the O-atom of the carbonyl group is treated as an “oxo” substituent and the molecule is called 2-Oxopropanoic acid Oxo-group ...
... For IUPAC naming of this structure, the O-atom of the carbonyl group is treated as an “oxo” substituent and the molecule is called 2-Oxopropanoic acid Oxo-group ...
Petasis reaction

The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the chemical reaction of an amine, aldehyde, and vinyl- or aryl-boronic acid to form substituted amines.Reported in 1993 by Nicos Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine, the Petasis reaction can be described as a variation of the Mannich reaction. Rather than generating an enolate to form the substituted amine product, in the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry and drug discovery.
![ch15[1].](http://s1.studyres.com/store/data/008194241_2-0a33cfb98ac502873dac865380b726e0-300x300.png)