jDonvert the following Fischer projections to dimensional formulas
... (a) Draw and show the difference in the energy diagrams of the rate determining step for both Bromine and Chlorine. (Be sure to show the relative energies of activation and whether the reactions are exothermic or ...
... (a) Draw and show the difference in the energy diagrams of the rate determining step for both Bromine and Chlorine. (Be sure to show the relative energies of activation and whether the reactions are exothermic or ...
投影片 1
... Active methylene compounds • Several active methylene compounds have been used as blocking agents; the reaction pathway differs from other blocked isocyanates since the dominant reaction with hydroxyl groups is to form esters rather than urethanes. ...
... Active methylene compounds • Several active methylene compounds have been used as blocking agents; the reaction pathway differs from other blocked isocyanates since the dominant reaction with hydroxyl groups is to form esters rather than urethanes. ...
Question number - Bethlehem College .::. Welcome
... HBr adds across the double bond in two ways to give two different products. This addition takes place according to Markovnikov’s rule (rich get richer). ...
... HBr adds across the double bond in two ways to give two different products. This addition takes place according to Markovnikov’s rule (rich get richer). ...
Aromatic Compounds
... Rings: The Friedel-Crafts Reaction Alkylation • The introduction of an alkyl group onto the benzene ring • Called the Friedel-Crafts reaction after its discoverers • Among the most useful electrophilic aromatic substitution ...
... Rings: The Friedel-Crafts Reaction Alkylation • The introduction of an alkyl group onto the benzene ring • Called the Friedel-Crafts reaction after its discoverers • Among the most useful electrophilic aromatic substitution ...
Sem-5 - NVPAS
... General introduction including nomenclature, General properties of terpenoids, Isolation, Isoprene rule, Classification of terpenoids, General methods for the determination of structure of terpenoids. Introduction, isolation and constitution of Citral, - terpineol, Geraniol, Nerol, Linalool. Refere ...
... General introduction including nomenclature, General properties of terpenoids, Isolation, Isoprene rule, Classification of terpenoids, General methods for the determination of structure of terpenoids. Introduction, isolation and constitution of Citral, - terpineol, Geraniol, Nerol, Linalool. Refere ...
Glycosyl amines
... roles in living matter. The most important are glycosyl amines derived from D-ribose or 2-deoxy-D-ribose and purine or pyrimidine beses (nucleosides), isolated from the hydrolyzates of nucleic acids. Another important group of glycosyl amines mediates the linkage between sugars and proteins in glyco ...
... roles in living matter. The most important are glycosyl amines derived from D-ribose or 2-deoxy-D-ribose and purine or pyrimidine beses (nucleosides), isolated from the hydrolyzates of nucleic acids. Another important group of glycosyl amines mediates the linkage between sugars and proteins in glyco ...
9851a doc..9851a chapter .. Page97
... Depending upon the synthetic route used, either halide ions or acid may remain in the ionic liquid, which can have dramatic effects upon catalytic reactions later performed in them. To remove these impurities it is necessary to dissolve the ionic liquid in dichloromethane (‘DCM’) and wash this solut ...
... Depending upon the synthetic route used, either halide ions or acid may remain in the ionic liquid, which can have dramatic effects upon catalytic reactions later performed in them. To remove these impurities it is necessary to dissolve the ionic liquid in dichloromethane (‘DCM’) and wash this solut ...
Full Article - PDF - Brandeis University
... alkaloid-catalyzed alcoholysis. Furthermore, the unreacted enantiomerically enriched UNCA (2) can be hydrolyzed to protected amino acid (4) (Scheme 1). The resulting mixture of the basic amine catalyst, the acidic amino acid (4) and the neutral amino ester (3), can be separated using simple extracti ...
... alkaloid-catalyzed alcoholysis. Furthermore, the unreacted enantiomerically enriched UNCA (2) can be hydrolyzed to protected amino acid (4) (Scheme 1). The resulting mixture of the basic amine catalyst, the acidic amino acid (4) and the neutral amino ester (3), can be separated using simple extracti ...
Chapter 2- Acids and Bases
... Right Left It cannot be determined. The forward and reverse reactions are equally favored. A ...
... Right Left It cannot be determined. The forward and reverse reactions are equally favored. A ...
Presence N-Methyl groups
... Presence of phenanthrene moiety 1)structure of methyl morphol 2)presence of N-Methyl group 3)position of 3 oxygen atoms 4)structure of morphenol 5)structure of mophine 6)point of linkage of –CH2-CH2-N-CH3 chain 7)position of double bond ...
... Presence of phenanthrene moiety 1)structure of methyl morphol 2)presence of N-Methyl group 3)position of 3 oxygen atoms 4)structure of morphenol 5)structure of mophine 6)point of linkage of –CH2-CH2-N-CH3 chain 7)position of double bond ...
Chapter 22 Alpha Substitution and Condensations of Enols
... • Tautomers are isomers which differ in the placement of a hydrogen. • One may be converted to the other. • In base: ...
... • Tautomers are isomers which differ in the placement of a hydrogen. • One may be converted to the other. • In base: ...
A-level Chemistry Question paper Unit 4 - Further Physical
... Total (Column 1) → Total (Column 2) → TOTAL ...
... Total (Column 1) → Total (Column 2) → TOTAL ...
lecture 3 - aldehydes and ketones
... covalent and the oxygen acquires a partial negative charge. Dipole/dipole interactions aren’t as strong as hydrogen bonds, but they do cause aldehydes and ketones to boil at higher temperatures than alkanes. ...
... covalent and the oxygen acquires a partial negative charge. Dipole/dipole interactions aren’t as strong as hydrogen bonds, but they do cause aldehydes and ketones to boil at higher temperatures than alkanes. ...
Spray Reagents - Murdercube.com
... The detection of chromatographic zones on a developed thin-layer plate usually relies on the absorption or emission of electromagnetic radiation in the visible or ultraviolet range. Some compounds are visibly coloured, others absorb UV light or exhibit Suorescence when excited by UV or visible light ...
... The detection of chromatographic zones on a developed thin-layer plate usually relies on the absorption or emission of electromagnetic radiation in the visible or ultraviolet range. Some compounds are visibly coloured, others absorb UV light or exhibit Suorescence when excited by UV or visible light ...
Chem 322 - Exam #3 - Spring 2003
... spaces provided in this booklet. This entire Exam Booklet must be handed in and will be returned to you with a grade. Write your name in the space above NOW. On the Test Scoring Answer Sheet, using a soft pencil, enter the following data (in the appropriate places): your name, instructor's name, you ...
... spaces provided in this booklet. This entire Exam Booklet must be handed in and will be returned to you with a grade. Write your name in the space above NOW. On the Test Scoring Answer Sheet, using a soft pencil, enter the following data (in the appropriate places): your name, instructor's name, you ...
Diphenylsilene - American Chemical Society
... preliminary results of a study of the chemistry of 1,l-diphenylsilene (2), which has been generated by photolysis of 1,1,2-triphenylsilacyclobutane (1) in polar and nonpolar solvents at room temperature and characterized using steady-state and nanosecond laser flash photolysis techniques. We also re ...
... preliminary results of a study of the chemistry of 1,l-diphenylsilene (2), which has been generated by photolysis of 1,1,2-triphenylsilacyclobutane (1) in polar and nonpolar solvents at room temperature and characterized using steady-state and nanosecond laser flash photolysis techniques. We also re ...
Isomers
... Rhodopsin is the opsin/cis-retinal combination, which is a light sensitive pigment. CN extends electron conjugation and allows it absorb visible light in the blue and green regions so rhodopsin looks purple in colour Other opsins produce cis-retinal complexes with different absorption spectra (colo ...
... Rhodopsin is the opsin/cis-retinal combination, which is a light sensitive pigment. CN extends electron conjugation and allows it absorb visible light in the blue and green regions so rhodopsin looks purple in colour Other opsins produce cis-retinal complexes with different absorption spectra (colo ...
The Ruff degradation: a review of previously proposed mechanisms
... organic solvents with both iron(III) porphyrin + periodate and manganese(III) porphyrin + periodate at room temperature.38–40 Investigations of the photoreduction of iron(III) porphyrins to iron(II) porphyrins have shown that a one-electron transfer from an axial ligand is involved. This mode of rea ...
... organic solvents with both iron(III) porphyrin + periodate and manganese(III) porphyrin + periodate at room temperature.38–40 Investigations of the photoreduction of iron(III) porphyrins to iron(II) porphyrins have shown that a one-electron transfer from an axial ligand is involved. This mode of rea ...
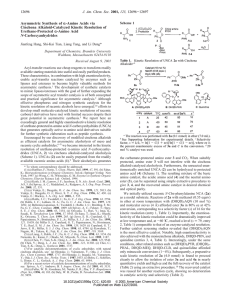
Chloroperbenzoic_aci..
... the allylic carbon (eq 4).18 Due to steric and other factors, the norbornene (11) undergoes selective (99%) epoxidation from the exo face.19 In 7,7-dimethylnorbornene (12), approach to the exo face is effectively blocked by the methyl substituent at C-7, and (12) is epoxidized from the unfavored end ...
... the allylic carbon (eq 4).18 Due to steric and other factors, the norbornene (11) undergoes selective (99%) epoxidation from the exo face.19 In 7,7-dimethylnorbornene (12), approach to the exo face is effectively blocked by the methyl substituent at C-7, and (12) is epoxidized from the unfavored end ...
Drug Metabolism
... • Typical N-substituents removed by oxidative dealkylation are methyl, ethyl, n-propyl, isopropyl, n-butyl, allyl, and benzyl • Dalkylation initially occurs with the smaller alkyl group. Substituents that are more resistant to dealkylation include the tert -butyl and the cyclopropylmethyl • Tertiary ...
... • Typical N-substituents removed by oxidative dealkylation are methyl, ethyl, n-propyl, isopropyl, n-butyl, allyl, and benzyl • Dalkylation initially occurs with the smaller alkyl group. Substituents that are more resistant to dealkylation include the tert -butyl and the cyclopropylmethyl • Tertiary ...
Chem 240 - Napa Valley College
... Although the general rule is to use the larger nuc- and the smaller substrate, doing so in this case would only lead to E2 elimination on #2. By using the smaller nuc- and larger substrate in #1 the reaction would go SN1 which would mean that you would get a lot of by-products but you would end up g ...
... Although the general rule is to use the larger nuc- and the smaller substrate, doing so in this case would only lead to E2 elimination on #2. By using the smaller nuc- and larger substrate in #1 the reaction would go SN1 which would mean that you would get a lot of by-products but you would end up g ...
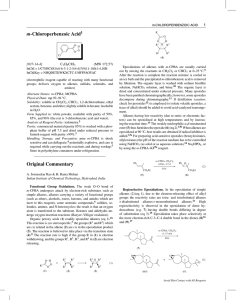
Petasis reaction

The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the chemical reaction of an amine, aldehyde, and vinyl- or aryl-boronic acid to form substituted amines.Reported in 1993 by Nicos Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine, the Petasis reaction can be described as a variation of the Mannich reaction. Rather than generating an enolate to form the substituted amine product, in the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry and drug discovery.