Organic Chemistry Fifth Edition
... In acid: protonate the oxygen, establishing the very good leaving group. More substituted carbon (more positive charge although more sterically hindered) is attacked by a weak nucleophile. Very similar to opening of cyclic bromonium ion. ...
... In acid: protonate the oxygen, establishing the very good leaving group. More substituted carbon (more positive charge although more sterically hindered) is attacked by a weak nucleophile. Very similar to opening of cyclic bromonium ion. ...
Chapter 4 Functional Group Transformations: Oxidation and
... Functional Group Transformations: Oxidation and Reduction Oxidation states (numbers) ...
... Functional Group Transformations: Oxidation and Reduction Oxidation states (numbers) ...
Organic Chemistry
... Carbon is unique in its ability to form long chains bonded to other carbon atoms, which results in a wide variety of chemical structures and unique organic compounds. With the exception of CH4, all hydrocarbons consist of a network of bonded C atoms. The structure of a hydrocarbon is often considere ...
... Carbon is unique in its ability to form long chains bonded to other carbon atoms, which results in a wide variety of chemical structures and unique organic compounds. With the exception of CH4, all hydrocarbons consist of a network of bonded C atoms. The structure of a hydrocarbon is often considere ...
Document
... 4. Reactions of condensation 1). Aldol condensation As noted earlier, an aldehyde is partially converted to its enolate anion by bases such as hydroxide ion and alkoxide ions. This type of condensations is character for aldehydes which have hydrogen atoms at the α-carbon atom. ...
... 4. Reactions of condensation 1). Aldol condensation As noted earlier, an aldehyde is partially converted to its enolate anion by bases such as hydroxide ion and alkoxide ions. This type of condensations is character for aldehydes which have hydrogen atoms at the α-carbon atom. ...
- Sacramento - California State University
... 1. Scheme 1: Synthesis of hydroxamic acid ligands for epoxidations in water.............. 14 2. Scheme 2: Synthesis and diastereomeric resolution of Mosher’s carboxylate ........... 22 3. Scheme 3: Analysis of solvent and epoxidation ......................................................... 27 4. Sc ...
... 1. Scheme 1: Synthesis of hydroxamic acid ligands for epoxidations in water.............. 14 2. Scheme 2: Synthesis and diastereomeric resolution of Mosher’s carboxylate ........... 22 3. Scheme 3: Analysis of solvent and epoxidation ......................................................... 27 4. Sc ...
$doc.title
... • Alcohols are weak nucleophiles but acid promotes addi3on forming the conjugate acid of C=O • Addi3on yields a hydroxy ether, called a hemiacetal (reversible); further reac3on can occur • Protona3on of the ...
... • Alcohols are weak nucleophiles but acid promotes addi3on forming the conjugate acid of C=O • Addi3on yields a hydroxy ether, called a hemiacetal (reversible); further reac3on can occur • Protona3on of the ...
Organic Chemistry - Rutgers University, Newark
... Conversion of an alcohol to a sulfonate ester converts HOH, a very poor leaving group, into a sulfonic ester, a very good leaving group ...
... Conversion of an alcohol to a sulfonate ester converts HOH, a very poor leaving group, into a sulfonic ester, a very good leaving group ...
Reactions of Alcohols
... The ZnCl2 coordinates to the hydroxyl oxygen, and this generates a far superior leaving group. Primary alcohols react in a similar fashion except the free cation is not generated, and the substitution is of S N2 ...
... The ZnCl2 coordinates to the hydroxyl oxygen, and this generates a far superior leaving group. Primary alcohols react in a similar fashion except the free cation is not generated, and the substitution is of S N2 ...
Dr.Farshid Zand Department of chemistry Organic Chem 233 L San
... (hydrochloric acid- zinc chloride) at 26 – 27 °C. Close the tube with a cork and shake; then allow the mixture to stand. Observe carefully for the first five minutes; land intermittently for up to one hour. Note the time required for reaction to take place, as indicted by the cloudy appearance of th ...
... (hydrochloric acid- zinc chloride) at 26 – 27 °C. Close the tube with a cork and shake; then allow the mixture to stand. Observe carefully for the first five minutes; land intermittently for up to one hour. Note the time required for reaction to take place, as indicted by the cloudy appearance of th ...
Manganese-Catalyzed Carbonylation of Alkyl Iodides
... without the possibility of further functionalization the method is not useful. There are a number of possible explanations for the exclusive formation of the protonated compounds: 1) The reaction of iodine with the biaryl zirconocene species may be significantly slower than competing protonation. 2) ...
... without the possibility of further functionalization the method is not useful. There are a number of possible explanations for the exclusive formation of the protonated compounds: 1) The reaction of iodine with the biaryl zirconocene species may be significantly slower than competing protonation. 2) ...
Aromatic Substitution Reactions
... ophile, in a fashion very similar to the addition reactions described in Chapter 11, which begin by reaction of an electrophile with the pi electrons of an alkene. This results in the formation of a carbocation called an arenium ion. Removal of a proton from the arenium ion by some weak base that is ...
... ophile, in a fashion very similar to the addition reactions described in Chapter 11, which begin by reaction of an electrophile with the pi electrons of an alkene. This results in the formation of a carbocation called an arenium ion. Removal of a proton from the arenium ion by some weak base that is ...
Identification of Alcohols
... molecule (H-O-H) has been replaced by an alkyl or substituted alkyl group. ...
... molecule (H-O-H) has been replaced by an alkyl or substituted alkyl group. ...
H - CashmereChemistry
... potassium dichromate it is converted to ethanoic acid, CH3COOH, a carboxylic acid. The equation for the oxidation of the primary alcohol ethanol is ...
... potassium dichromate it is converted to ethanoic acid, CH3COOH, a carboxylic acid. The equation for the oxidation of the primary alcohol ethanol is ...
Organic Chemistry
... biological and chemical processes which can be prepared by the oxidation of thiols. For this work, we have developed a new preparative method for the disulfide using inexpensive, recyclable, and relatively non-toxic polymer-supported (diacetoxyiodo)benzene (PS-DIB) as the oxidant under mild conditio ...
... biological and chemical processes which can be prepared by the oxidation of thiols. For this work, we have developed a new preparative method for the disulfide using inexpensive, recyclable, and relatively non-toxic polymer-supported (diacetoxyiodo)benzene (PS-DIB) as the oxidant under mild conditio ...
How does it vary with the charge and distance of the ions?
... 2NO2 (slow). Derive the rate law for K-1 the process. m) How does equivalent conductance vary with concentration in case of both strong and weak electrolyte? n) State Kohlrausch’s law of independent migration of ion. Using this law, how will you get equivalent conductance of weak electrolyte at infi ...
... 2NO2 (slow). Derive the rate law for K-1 the process. m) How does equivalent conductance vary with concentration in case of both strong and weak electrolyte? n) State Kohlrausch’s law of independent migration of ion. Using this law, how will you get equivalent conductance of weak electrolyte at infi ...
- kunleoloruntegbe.com
... Alchohol addition: Alkanals, but not alkanones, will give addition reactions with alcohols provided all the reagent are dry, and that Hydrochloric acid (HCL) is used to catalyse the reaction. The most common example of this type of addition is ethanol additing to ethanal. dry CH3CHO + 2C2 H5 OH HCl ...
... Alchohol addition: Alkanals, but not alkanones, will give addition reactions with alcohols provided all the reagent are dry, and that Hydrochloric acid (HCL) is used to catalyse the reaction. The most common example of this type of addition is ethanol additing to ethanal. dry CH3CHO + 2C2 H5 OH HCl ...
8. Alkynes: An Introduction to Organic Synthesis
... elimination of HX (double dehydrohalogenation) Vicinal dihalides are available from addition of bromine or chlorine to an ...
... elimination of HX (double dehydrohalogenation) Vicinal dihalides are available from addition of bromine or chlorine to an ...
ALCOHOLS AND ETHERS
... expected to be similar to those of water, H O H , the simplest hydroxylic compound. Alcohols, ROH, can be regarded in this respect as substitution products of water. However, with alcohols we shall be interested not only in reactions that proceed at the 8-H bond but also with processes that result i ...
... expected to be similar to those of water, H O H , the simplest hydroxylic compound. Alcohols, ROH, can be regarded in this respect as substitution products of water. However, with alcohols we shall be interested not only in reactions that proceed at the 8-H bond but also with processes that result i ...
Formation of Benzoic Acid
... 19. Using a glass funnel and filter paper, gravity filter the contents of the beaker into another clean, small beaker. Add about 2 mL of water to this beaker that contained the NaOH layers from Step 18, swirl around, rinsing the inside of the beaker as best as possible. Pour this through the filter ...
... 19. Using a glass funnel and filter paper, gravity filter the contents of the beaker into another clean, small beaker. Add about 2 mL of water to this beaker that contained the NaOH layers from Step 18, swirl around, rinsing the inside of the beaker as best as possible. Pour this through the filter ...
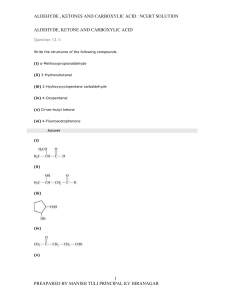
carbonyl compound group
... (vi) Oxime: Oximes are a class of organic compounds having the general formula RR′CNOH, where R is an organic side chain and R′ is either hydrogen or an organic side chain. If R′ is H, then it is known as aldoxime and if R′ is an organic side chain, it is known as ketoxime. ...
... (vi) Oxime: Oximes are a class of organic compounds having the general formula RR′CNOH, where R is an organic side chain and R′ is either hydrogen or an organic side chain. If R′ is H, then it is known as aldoxime and if R′ is an organic side chain, it is known as ketoxime. ...
Improved Synthesis of (3E,6Z,9Z)-1,3,6,9
... The winter moth, Operophtera brumata (Lepidoptera: Geometridae), is an early-season defoliator that attacks a wide variety of hardwoods and, in some cases, conifers. The insect is native to Europe but has become established in at least three areas of North America including southeastern New England. ...
... The winter moth, Operophtera brumata (Lepidoptera: Geometridae), is an early-season defoliator that attacks a wide variety of hardwoods and, in some cases, conifers. The insect is native to Europe but has become established in at least three areas of North America including southeastern New England. ...
Amidations of Rosin with Isocyanates
... for the addition amounts of the catalysts between 10 and 20 mg/g abietic acid. The results demonstrate that the tertiary amines can greatly accelerate the reaction rate of abietic acid with phenyl isocyanate. Amidation of rosin with phenyl isocyanate Table 3 shows the results of amidation of rosin w ...
... for the addition amounts of the catalysts between 10 and 20 mg/g abietic acid. The results demonstrate that the tertiary amines can greatly accelerate the reaction rate of abietic acid with phenyl isocyanate. Amidation of rosin with phenyl isocyanate Table 3 shows the results of amidation of rosin w ...
Petasis reaction

The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the chemical reaction of an amine, aldehyde, and vinyl- or aryl-boronic acid to form substituted amines.Reported in 1993 by Nicos Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine, the Petasis reaction can be described as a variation of the Mannich reaction. Rather than generating an enolate to form the substituted amine product, in the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry and drug discovery.