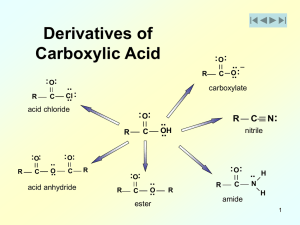
Answers
... oxidized further to the caboxylic acid while propanone is not easily oxidized. The Tollens’ Test for aldehydes makes use of this difference in properties. (Tollens’ reagent is made by adding sodium hydroxide to silver nitrate solution to give a precipitate of silver hydroxide and then redissolving t ...
... oxidized further to the caboxylic acid while propanone is not easily oxidized. The Tollens’ Test for aldehydes makes use of this difference in properties. (Tollens’ reagent is made by adding sodium hydroxide to silver nitrate solution to give a precipitate of silver hydroxide and then redissolving t ...
T II Structure and Properties of the Macronutrients U N I T
... ketose forms a cyclic hemiacetal or cyclic hemiketal, which are also called lactols. When a ring structure forms, the carbonyl carbon is transformed into a chiral carbon, giving rise to a new pair of isomers called α- and β-anomers. Aldoses and ketoses are more stable in their five- or six-membered c ...
... ketose forms a cyclic hemiacetal or cyclic hemiketal, which are also called lactols. When a ring structure forms, the carbonyl carbon is transformed into a chiral carbon, giving rise to a new pair of isomers called α- and β-anomers. Aldoses and ketoses are more stable in their five- or six-membered c ...
Sample Chapter 04 - Structure and Properties of the
... ketose forms a cyclic hemiacetal or cyclic hemiketal, which are also called lactols. When a ring structure forms, the carbonyl carbon is transformed into a chiral carbon, giving rise to a new pair of isomers called α- and β-anomers. Aldoses and ketoses are more stable in their five- or six-membered c ...
... ketose forms a cyclic hemiacetal or cyclic hemiketal, which are also called lactols. When a ring structure forms, the carbonyl carbon is transformed into a chiral carbon, giving rise to a new pair of isomers called α- and β-anomers. Aldoses and ketoses are more stable in their five- or six-membered c ...
Heterogeneous Catalysts for Biodiesel Production
... separation because of emulsion formation.3,6 Several methods have been proposed to solve these problems,11 but the most useful seem to be the following:5,10,12–15 (a) use of enzymes. A Lypase enzyme catalyzes both transesterification of triglycerides and esterification of FFA R-COOH + MeOH a R-COOMe ...
... separation because of emulsion formation.3,6 Several methods have been proposed to solve these problems,11 but the most useful seem to be the following:5,10,12–15 (a) use of enzymes. A Lypase enzyme catalyzes both transesterification of triglycerides and esterification of FFA R-COOH + MeOH a R-COOMe ...
Sample Midterm 1B
... 2. a. (1 Mark) Butanol, C4H9OH, Diethyl Ether CH3CH2OCH2CH3 and Pentane C5H12 all have similar molecular weights but Butanol has a much higher boiling point. What property of butanol would explain this boiling point difference? ...
... 2. a. (1 Mark) Butanol, C4H9OH, Diethyl Ether CH3CH2OCH2CH3 and Pentane C5H12 all have similar molecular weights but Butanol has a much higher boiling point. What property of butanol would explain this boiling point difference? ...
lecture 11 catalysis_hydrogenation of alkenes
... The protons of a dihydrogen ligand are known to be more acidic than those of free H2, and many H2 complexes can be deprotonated by NEt3. ...
... The protons of a dihydrogen ligand are known to be more acidic than those of free H2, and many H2 complexes can be deprotonated by NEt3. ...
Preparation of d, l-Phenylalanine by Amidocarbonylation of Benzyl
... acids is Wakamatsu's amidocarbonylation reaction3,4 which allows the preparation of amino acids from aldehydes containing at least one R-proton, CO, and a limited class of amides, in particular acetamide. The necessity of the R-proton is best explained by assuming the intermediacy of the enamide (Sc ...
... acids is Wakamatsu's amidocarbonylation reaction3,4 which allows the preparation of amino acids from aldehydes containing at least one R-proton, CO, and a limited class of amides, in particular acetamide. The necessity of the R-proton is best explained by assuming the intermediacy of the enamide (Sc ...
Slide 1
... the acid and alcohol in the presence of a small quantity of acid catalyst (H2SO4 or HCl (g)) causes ester formation (esterification) along with dehydration. The equilibrium constant is not large (Keq ~ 1) but high yields can be obtained by adding a large excess of one of the reactants and removing t ...
... the acid and alcohol in the presence of a small quantity of acid catalyst (H2SO4 or HCl (g)) causes ester formation (esterification) along with dehydration. The equilibrium constant is not large (Keq ~ 1) but high yields can be obtained by adding a large excess of one of the reactants and removing t ...
No Slide Title
... material found in blood clots. Unregulated clotting may lead to cardiac arrest or stroke. • A Peptidomimetic is any small organic molecule that mimics the transition state of a natural substrate. • Peptidomimetics competitively inhibit the enzyme process, preventing the natural reaction from occurri ...
... material found in blood clots. Unregulated clotting may lead to cardiac arrest or stroke. • A Peptidomimetic is any small organic molecule that mimics the transition state of a natural substrate. • Peptidomimetics competitively inhibit the enzyme process, preventing the natural reaction from occurri ...
ether - HCC Southeast Commons
... Practice Problem: Why do you suppose only symmetrical ethers are prepared by the sulfuric acid-catalyzed dehydration procedure? What product(s) would you expect if ethanol and 1-propanol were allowed to react together? In what ratio would the products be formed if the two alcohols were of equal rea ...
... Practice Problem: Why do you suppose only symmetrical ethers are prepared by the sulfuric acid-catalyzed dehydration procedure? What product(s) would you expect if ethanol and 1-propanol were allowed to react together? In what ratio would the products be formed if the two alcohols were of equal rea ...
Aromatic electrophilic substitution
... substituent groups because the individual effects are mutually supporting of each other. 3. In cases were there is a conflict in the directing effects of the substituent groups it can more difficult to predict what products will be produced. When dealing with multiple substituents activating groups ...
... substituent groups because the individual effects are mutually supporting of each other. 3. In cases were there is a conflict in the directing effects of the substituent groups it can more difficult to predict what products will be produced. When dealing with multiple substituents activating groups ...
Cerium(IV) Ammonium Nitrate as a Catalyst in
... UCM in 1988, under the supervision of Dr. Mónica M. Söllhuber. In August 1988, he joined the group of Professor Steven V. Ley at Imperial College, London, where he worked on the total synthesis of the natural ionophoric antibiotic routiennocin. In September 1989, he returned as a Profesor Titular ...
... UCM in 1988, under the supervision of Dr. Mónica M. Söllhuber. In August 1988, he joined the group of Professor Steven V. Ley at Imperial College, London, where he worked on the total synthesis of the natural ionophoric antibiotic routiennocin. In September 1989, he returned as a Profesor Titular ...
PDF - Nanyang Technological University
... electron-deficient anilines were unsuitable substrates for the oxidative reactions and products 6 c and 6 d were not obtained (Scheme 1). With aliphatic cyclic amines, the size of the ring was found to affect the outcome of the reaction. The reaction of an N-aryl pyrrolidine with 2 a gave the desire ...
... electron-deficient anilines were unsuitable substrates for the oxidative reactions and products 6 c and 6 d were not obtained (Scheme 1). With aliphatic cyclic amines, the size of the ring was found to affect the outcome of the reaction. The reaction of an N-aryl pyrrolidine with 2 a gave the desire ...
Document
... hydride (DIBAlH) at -78°C selectively reduces an ester to an aldehyde • at -78°C, the TCAI does not collapse and it is not until hydrolysis in aqueous acid that the carbonyl group of the aldehyde is liberated O ...
... hydride (DIBAlH) at -78°C selectively reduces an ester to an aldehyde • at -78°C, the TCAI does not collapse and it is not until hydrolysis in aqueous acid that the carbonyl group of the aldehyde is liberated O ...
Chapter 16
... This fact is actually fortunate because otherwise it would be difficult to form Grignard reagents since the Grignard is a result of an alkyl halide reacting with magnesium ...
... This fact is actually fortunate because otherwise it would be difficult to form Grignard reagents since the Grignard is a result of an alkyl halide reacting with magnesium ...
Presentation
... Grignard reagent is converted into 1° alcohol using H−CHO or ethyleneoxide 1° alcohol containing 2 carbon atoms more than the Grignard reagent can be obtained by treating with R-Mg-X with ethylene oxide followed by acid hydrolysis, ...
... Grignard reagent is converted into 1° alcohol using H−CHO or ethyleneoxide 1° alcohol containing 2 carbon atoms more than the Grignard reagent can be obtained by treating with R-Mg-X with ethylene oxide followed by acid hydrolysis, ...
Chapters E-18 review - Bakersfield College
... 4. Show thé acid, base, conjugate acid and conjugale base in following reactions: ...
... 4. Show thé acid, base, conjugate acid and conjugale base in following reactions: ...
Nuggets of Knowledge for Chapter 12 – Alcohols
... o Acid-catalyzed hydration of alkenes, accomplished by treating an alkene with a catalytic amount of sulfuric or phosphoric acid and water (Ch 9) o Oxymercuration-reduction of alkenes, accomplished by treating an alkene with mercuric acetate and water, followed by sodium borohydride. (Ch 10) o Hydro ...
... o Acid-catalyzed hydration of alkenes, accomplished by treating an alkene with a catalytic amount of sulfuric or phosphoric acid and water (Ch 9) o Oxymercuration-reduction of alkenes, accomplished by treating an alkene with mercuric acetate and water, followed by sodium borohydride. (Ch 10) o Hydro ...
Petasis reaction

The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the chemical reaction of an amine, aldehyde, and vinyl- or aryl-boronic acid to form substituted amines.Reported in 1993 by Nicos Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine, the Petasis reaction can be described as a variation of the Mannich reaction. Rather than generating an enolate to form the substituted amine product, in the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry and drug discovery.