Organic Acids and Bases and Some of Their Derivatives
... like an ether and also somewhat like a carboxylic acid. Even so, compounds in this group react neither like carboxylic acids nor like ethers; they make up a distinctive family. Unlike ethers, esters have a carbonyl group. Unlike carboxylic acids, esters have no acidic hydrogen atom; they have a hydr ...
... like an ether and also somewhat like a carboxylic acid. Even so, compounds in this group react neither like carboxylic acids nor like ethers; they make up a distinctive family. Unlike ethers, esters have a carbonyl group. Unlike carboxylic acids, esters have no acidic hydrogen atom; they have a hydr ...
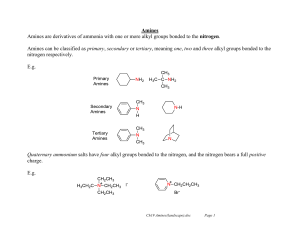
Amines Amines are derivatives of ammonia with one or more alkyl
... Pyridine behaves like a strongly deactivated aromatic compound in EAS reactions. FC alkylations and acetylations fail, and other EAS reactions require unusually harsh reaction conditions. The deactivation arises from the electron withdrawing effect of the nitrogen atom in the ring. The lone pair of ...
... Pyridine behaves like a strongly deactivated aromatic compound in EAS reactions. FC alkylations and acetylations fail, and other EAS reactions require unusually harsh reaction conditions. The deactivation arises from the electron withdrawing effect of the nitrogen atom in the ring. The lone pair of ...
FULL PAPER Observations on the Influence of Precursor
... subjected to the Swern oxidation conditions 24 to afford the corresponding crude aldehyde followed by oxidation to acid 11 using NaClO2, NaH2PO4, and 2-methylbut-2-ene25 in 87% yield. The synthetic route to compound 21 started with treatment of commercially available (4-methoxyphenyl)methanol (12) a ...
... subjected to the Swern oxidation conditions 24 to afford the corresponding crude aldehyde followed by oxidation to acid 11 using NaClO2, NaH2PO4, and 2-methylbut-2-ene25 in 87% yield. The synthetic route to compound 21 started with treatment of commercially available (4-methoxyphenyl)methanol (12) a ...
IB2 Revision Topic 7
... The amount of SO3 and the value of the equilibrium constant both increase. The amount of SO3 and the value of the equilibrium constant both decrease. The amount of SO3 increases but the value of the equilibrium constant decreases. The amount of SO3 increases but the value of the equilibrium constant ...
... The amount of SO3 and the value of the equilibrium constant both increase. The amount of SO3 and the value of the equilibrium constant both decrease. The amount of SO3 increases but the value of the equilibrium constant decreases. The amount of SO3 increases but the value of the equilibrium constant ...
A GRIGNARD REACTION: SYNTHESIS OF 2-METHYL-2
... C bearing the halogen is electron-poor (halogens are more electronegative than C). That C changes to electron-rich once connected to Mg (which is less electronegative than C). This change is sometimes called “Umpolung” – German for “change in polarity”. 2. The Grignard reagent is mixed with a compou ...
... C bearing the halogen is electron-poor (halogens are more electronegative than C). That C changes to electron-rich once connected to Mg (which is less electronegative than C). This change is sometimes called “Umpolung” – German for “change in polarity”. 2. The Grignard reagent is mixed with a compou ...
Chapter 7 Test Version A
... 1. A solution that produces the hydroxide ion is called a(n) __________________ 2. In a mixture, the stuff that is dissolved by the other stuff is called the ____________________ 3. In a solution the stuff that dissolves the other stuff is called the ________________. 4. If a solution has a pH of 8 ...
... 1. A solution that produces the hydroxide ion is called a(n) __________________ 2. In a mixture, the stuff that is dissolved by the other stuff is called the ____________________ 3. In a solution the stuff that dissolves the other stuff is called the ________________. 4. If a solution has a pH of 8 ...
Development of New Synthetic Routes to Organoboronates by Catalytic Allylic Substitution and
... the classical methods for the synthesis of allylboronate derivatives are often associated with problems, such as low regio- and stereoselectivity as well as poor functional group tolerance, milder methods are required for obtaining functionalized derivatives. The use of transition metal catalysis ha ...
... the classical methods for the synthesis of allylboronate derivatives are often associated with problems, such as low regio- and stereoselectivity as well as poor functional group tolerance, milder methods are required for obtaining functionalized derivatives. The use of transition metal catalysis ha ...
Oxidation of Diols and Ethers by NaBr03
... molar amounts of NaBr03/NaHS03, 2-bromo-l,3-cyclohexanedione 22 was obtained in preference to the expected 1,3cyclohexanedione (40) (Table 2, Run 2). A plausible reaction path for the production of 22 from 19 is shown in Scheme 1. In a previous paper, we showed that enones upon treatment with NaBr03 ...
... molar amounts of NaBr03/NaHS03, 2-bromo-l,3-cyclohexanedione 22 was obtained in preference to the expected 1,3cyclohexanedione (40) (Table 2, Run 2). A plausible reaction path for the production of 22 from 19 is shown in Scheme 1. In a previous paper, we showed that enones upon treatment with NaBr03 ...
Chapter 1 Chirality in clinical analysis 1.1. Introduction
... administrated as racemates [31, 32]. Although the S or R isomer has the same substituent atoms or groups, qualitatively or quantitatively may have similar or different pharmacological effects, which may relate to their stereoselective pharmacokinetics or pharmacodynamics. The terms “eutomer” for the ...
... administrated as racemates [31, 32]. Although the S or R isomer has the same substituent atoms or groups, qualitatively or quantitatively may have similar or different pharmacological effects, which may relate to their stereoselective pharmacokinetics or pharmacodynamics. The terms “eutomer” for the ...
Ethers and Epoxides
... • Simple ethers are named by identifying the two organic substituents and adding the word ether • If other functional groups are present, the ether part is considered an alkoxy substituent ...
... • Simple ethers are named by identifying the two organic substituents and adding the word ether • If other functional groups are present, the ether part is considered an alkoxy substituent ...
- Thieme Connect
... Triphenylphosphine dibromide (TPPDB, PPh3Br2), originally synthesized by Horner and his co-workers,1 has shown significant synthetic versatility over the course of the last decades in organic synthesis. It has been used extensively in various organic transformations, such as bromination of alcohols, ...
... Triphenylphosphine dibromide (TPPDB, PPh3Br2), originally synthesized by Horner and his co-workers,1 has shown significant synthetic versatility over the course of the last decades in organic synthesis. It has been used extensively in various organic transformations, such as bromination of alcohols, ...
Carbohydrates
... • Comparing the straight chain and cyclic forms of monosaccharides, the OH groups that are located on the right of a chiral center point down in the cyclic form. C-5 ...
... • Comparing the straight chain and cyclic forms of monosaccharides, the OH groups that are located on the right of a chiral center point down in the cyclic form. C-5 ...
Year 2 Chemistry Contents Guide
... Reactions of amines 13 slides • Aliphatic and aromatic amines Animation illustrating the action of amines as Brønsted–Lowry bases • Relative base strength of the amines, and the reactions of amines as bases to form salts Animation illustrating the action of amines as nucleophiles: reaction with halo ...
... Reactions of amines 13 slides • Aliphatic and aromatic amines Animation illustrating the action of amines as Brønsted–Lowry bases • Relative base strength of the amines, and the reactions of amines as bases to form salts Animation illustrating the action of amines as nucleophiles: reaction with halo ...
Organic Chemistry II
... Organic synthesis is very important in that it allows the experimenter to make new compounds from compounds that might be more readily available. Sometimes a synthesis reaction is easy to do and other times great effort and care must be given. This experiment will require good technique as the cyclo ...
... Organic synthesis is very important in that it allows the experimenter to make new compounds from compounds that might be more readily available. Sometimes a synthesis reaction is easy to do and other times great effort and care must be given. This experiment will require good technique as the cyclo ...
Derivatization Reagents - Sigma
... their methyl esters. Hydroxyl groups are not methylated. Carboxylic acids, phenols, and thiols react quickly, to give the corresponding alkyl derivatives. N,N-dimethylformamide dimethylacetals are moisture sensitive. ...
... their methyl esters. Hydroxyl groups are not methylated. Carboxylic acids, phenols, and thiols react quickly, to give the corresponding alkyl derivatives. N,N-dimethylformamide dimethylacetals are moisture sensitive. ...
Chapter 2 Phenols
... 5- Reactions of Phenols A hydroxyl group is very powerful activating substituent, and electrophilic aromatic substitution in phenol occurs far faster, and under milder condition, than in benzene. a- Halogenation ...
... 5- Reactions of Phenols A hydroxyl group is very powerful activating substituent, and electrophilic aromatic substitution in phenol occurs far faster, and under milder condition, than in benzene. a- Halogenation ...
enantioselective zeolite-catalyzed reactions
... major topic of discussion and research.1 Homogeneous, asymmetry-inducing catalysts have been developed for a wide range of reactions, especially notable are those developed by Knowles, Noyori and Sharpless, for which they jointly received the 2001 Nobel Prize in Chemistry.2 Although these reactions ...
... major topic of discussion and research.1 Homogeneous, asymmetry-inducing catalysts have been developed for a wide range of reactions, especially notable are those developed by Knowles, Noyori and Sharpless, for which they jointly received the 2001 Nobel Prize in Chemistry.2 Although these reactions ...
Petasis reaction

The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the chemical reaction of an amine, aldehyde, and vinyl- or aryl-boronic acid to form substituted amines.Reported in 1993 by Nicos Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine, the Petasis reaction can be described as a variation of the Mannich reaction. Rather than generating an enolate to form the substituted amine product, in the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry and drug discovery.