* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Isomers
Survey
Document related concepts
Transcript
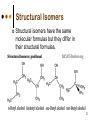
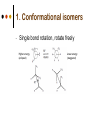
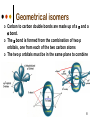
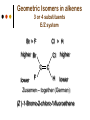
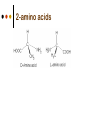
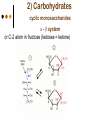
Isomers 1 Structural Isomers Structural isomers have the same molecular formulas but they differ in their structural formulas. 2 Structural Isomers C4H10O C6H14 C7H16 C6H12 3 Stereoisomers Stereo isomers have the same structural formulas but they differ in their spatial arrangements. There are two types of stereoisomerism 1. 2. Conformational isomerism Configurational isomerism 4 1. Conformational isomers - Single bond rotation, rotate freely Higher energy (eclipsed) Lower energy (staggered) 2. Configurational Isomers Interconverted only by the breaking of bonds There are 2 types of configurational isomers A. Geometric (cis/trans, E/Z) B. Optical isomers A. Geometrical isomers Geometrical isomers occur in organic molecules where rotation around a bond is restricted This occurs most often around C=C The most common cases are around asymmetric non-cyclic alkenes 7 Geometrical isomers Carbon to carbon double bonds are made up of a s and a p bond. The p bond is formed from the combination of two p orbitals, one from each of the two carbon atoms The two p orbitals must be in the same plane to combine 8 Geometric Isomers in alkenes 2 substituents cis/trans system A cis isomer is one in which the substituents are on the same side of the C=C • cis – 2 – butene A trans isomer is one in which the substituents are on the opposite sides of the C=C trans – 2 – butene 9 Geometric Isomers in alkenes 3 or 4 substituents E/Z system CIP system ranks according to atomic number, higher atomic number outranks lower atomic number. E : higher ranked substituents on opposite sides Z : higher ranked substituents on same side Geometric Isomers in alkenes 3 or 4 substituents E/Z system Br > F Cl > H higher Br C lower F Cl higher H lower C Zusamen – together (German) (Z )-1-Bromo-2-chloro-1-fluoroethene Geometric Isomers in alkenes 3 or 4 substituents E/Z system Br > F lower Cl > H F C higher Br Cl higher H lower C Etgegen – opposite (German) (E)-1-Bromo-2-chloro-1-fluoroethene Geometric Isomers in alkenes 3 or 4 substituents E/Z systemles When two atoms are identical, compare the atoms attached to them on the basis of their atomic numbers. Precedence is established at the first point of difference. —CH2CH3 —C(C,H,H) outranks —CH3 —C(H,H,H) Geometric Isomers in alkenes 3 or 4 substituents E/Z system Evaluate substituents one by one. Don't add atomic numbers within groups. —CH2OH outranks —C(CH3)3 —C(O,H,H) —C(C,C,C) Geometric Isomers in Cycloalkanes Ring structures like C=C restrict rotation and therefore can result in cis and trans isomers 15 Properties of Geometrical Isomers The chemical properties of geometrical isomers tend to be similar but their physical properties are different 16 Properties of Geometrical Isomers The trans isomer has a much higher melting point. Unlike the cis isomer there is little intra-molecular hydrogen bonding 17 B. Optical Isomers Optical isomerism is present in all compounds that contain at least one asymmetric (chiral) carbon atom An asymmetric carbon atom has four different atoms or groups attached In this case there are two different ways to arrange the four groups around the chiral carbon atom (shown in red) 18 Optical Isomers While these structures may look identical, in three dimensions they are mirror images of each other. Such molecules are called enantiomers. 19 Distinguishing Enantiomers Optical isomers can be distinguished by the way they interact with plane polarized light Polarized light Polarized light is light that has been passed through a polarizing prism or filter. As a result the light vibrates in a single plane. Distinguishing Enantiomers Optical isomers can be distinguished by the way they interact with plane polarized light. One enantiomer will rotate polarized light to the right, the other to the left. Racemic mixture equal amounts of both enantiomers does not alter light Properties of Optical Isomers Apart from their optical activity enantiomers generally have similar physical and chemical properties. The chemical properties may be significantly different when the enantiomers interact with other optically active compounds. Optical isomer limonene (+) isomer strong smell of oranges/lemons (-) isomer strong smell of pine needles/peppermint Optical isomer Thalidomide (+) isomer is a sedative (-) isomer is a teratogen B.10 Stereochemistry in Biomolecules Stereochemistry of biological molecules 1) 2-amino acids 2) Carbohydrate 3) Fatty acids 4) Retinoids 1) 2 – amino acids - - All amino acids have a chiral centre except glycine. All amino acid enantiomers are Lisomers wedge-dash fischer projections 2-amino acids Fischer projections are drawn as a cross with the carbon chain vertically, so at the top is COOH (amino acids) or C=O (carbohydrates) amino acids carbohydrates 2-amino acids L – enantiomers have amino group on the left D – enantiomers have amino group on the right 2-amino acids - Other method for identifying enantiomers is called the CORN rule (COOH, R and NH2) - Position molecule so H atom at C-2 faces away - CO – R – N is spelled clockwise you have D – enantiomer - CO – R – N is spelled anti-clockwise it is L – enantiomer 2-amino acids 2) Carbohydrates - For the D and L system the C=O is at the top and you look on which side the OH is on the chiral carbon furthest from the C=O. 2) Carbohydrates Enantiomers – mirror images Diastereomers – when 2 stereoisomers of a compound have different configurations at one or more (but not all) chiral centres Epimers – when 2 diastereomers differ at only 1 chiral centre. 2) Carbohydrates cyclic monosaccharides - system Each monosaccharide can produce 2 cyclic forms that differ in the orientation of the –OH group at C-1 atom in glucose (aldoses = aldehyde) 2) Carbohydrates cyclic monosaccharides - system or C-2 atom in fructose (ketoses = ketone) 2) Carbohydrates cyclic monosaccharides - system - tend to be more water soluble The conversion b/n and occurs through open chain isomer 2) Carbohydrates Cellulose - system - - Cellulose is a polymer of -glucose, most abundant polysaccharides in plants. Glucose residues are joined by -1,4glycosidic links 2) Carbohydrates Cellulose - system - - - Starch and glycogen are polymers of -glucose and are branched polymers No branching in cellulose causes stronger intermolecular forces (Hbonds) causing insolubility in water, stronger and lower dietary value Humans and animals cannot digest cellulose. Ruminants contain bacteria that produce cellulase in their digestive system 2) Carbohydrates Cellulose - system - Common name is dietary fibre, can’t be digested but - - - Cleans and prevents constipation Reduces appetite Helps prevent obesity Regulates absorption of sugars and bile Reducing diabetes and cholesterol 3) Fatty acids and triglycerides - system - - - - naturally occuring fatty acids have cis C=C, so not linear, weaker intermolecular forces, therefore cisfats liquids at room temperature Hydrogenation (similar to addition of alkenes) turns cis-fats into saturated fats. Requires high temp. and nickel catalyst. High temp. of process leads to some cis-fats becoming trans-fats 3) Fatty acids and triglycerides - system - High temp. of process leads to some cis-fats becoming trans-fats - Melting points are higher than cis-fats and lower than saturated fats of equal length. 3) Fatty acids and triglycerides - system - - - - Trans-fats trace amounts present naturally occuring. 15% in margarine (man-made) High in fast food industry High consumption leads to high LDL increases risk of heart disease Difficult to metabolize stored in fat increased obesity Links to diabetes and Alzheimer’s 4) Retinoids Vitamin A - - Vitamin A refers to a group of polyunsaturated compounds, one of which, retinal (a long chain aldehyde) is involved in vision chemistry. In the photoreceptor cells 2 stereoisomers for retinal (cis-retinal and trans-retinal) which can be converted into each other with visible light or enzymes 4) Retinoids Vitamin A - - - - Opsin is a protein that can reversibly bind cisretinal Rhodopsin is the opsin/cis-retinal combination, which is a light sensitive pigment. CN extends electron conjugation and allows it absorb visible light in the blue and green regions so rhodopsin looks purple in colour Other opsins produce cis-retinal complexes with different absorption spectra (colour vision) 4) Retinoids Vitamin A - - - Whe rhodopsin absorbs a photon of visible light, the residue of cis-retinal turns into trans-retinal and the protein conformation changes. This triggers a cell response that sends electrical signal to nervous system. Trans-retinal detaches from opsin goes through visual cycle and converts back to cis-retinal and recombines with opsin to reform rhodopsin