Synthesis of enantiopure alcohols
... transesterification reactions of secondary alcohols catalyzed by a pure protein formulation of lipase B from Candida antarctica (Novozym 525 F). Addition of a range of enantiopure alcohols caused a temporary increase in enzyme selectivity in the transesterification reaction of 3-chloro-1-phenoxy-2-p ...
... transesterification reactions of secondary alcohols catalyzed by a pure protein formulation of lipase B from Candida antarctica (Novozym 525 F). Addition of a range of enantiopure alcohols caused a temporary increase in enzyme selectivity in the transesterification reaction of 3-chloro-1-phenoxy-2-p ...
Asymmetric catalytic routes to chiral building blocks of
... general, enantioselective method for the reduction of p-keto esters (16) to p-hydroxy esters (17) under mild conditions (MeOWH20 (9/1), 35 OC, 60 psi H2, SIC = 500, 20 h). For example, hydrogenation with the (R,R)-i-Pr-BPE-Ru catalyst afforded a variety of linear and branched alkyl-substituted phydr ...
... general, enantioselective method for the reduction of p-keto esters (16) to p-hydroxy esters (17) under mild conditions (MeOWH20 (9/1), 35 OC, 60 psi H2, SIC = 500, 20 h). For example, hydrogenation with the (R,R)-i-Pr-BPE-Ru catalyst afforded a variety of linear and branched alkyl-substituted phydr ...
Mon Feb 15 lecture
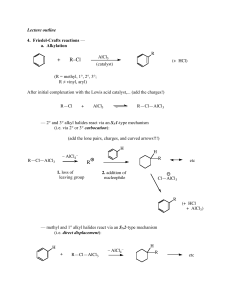
... (This is frequently observed in carbocation chemistry; we've already seen a similar concerted rearrangement and leaving group (water) departure in the reaction of some 1° alcohols with strong acid.) not Cl ...
... (This is frequently observed in carbocation chemistry; we've already seen a similar concerted rearrangement and leaving group (water) departure in the reaction of some 1° alcohols with strong acid.) not Cl ...
Number of Electron Pairs Allowed Sigmatropic Rearrangement
... Examples of Sigmatropic Rearrangements The [1,3] sigmatropic rearrangement is photochemically allowed. ...
... Examples of Sigmatropic Rearrangements The [1,3] sigmatropic rearrangement is photochemically allowed. ...
KINETIC AND MECHANISTIC STUDY OF OXIDATION OF ESTER
... negligible effect on the rate. For reactions in solution the nature of solvent plays an important role which has been discussed in detail by Aims . In present investigation, effect of solvent could not be studies because of reactivity of solvent such as alcohol ...
... negligible effect on the rate. For reactions in solution the nature of solvent plays an important role which has been discussed in detail by Aims . In present investigation, effect of solvent could not be studies because of reactivity of solvent such as alcohol ...
3672 been studied in detail by Kebarle, et al., who
... priori, lead to the pentacoordinate phosphorane 1 and (or) the phosphonium salts 2-4 which in turn may lead to either sulfides (collapse of 3) or ethers (collapse of 2 or 4).9 However, we have found that only products which appear to be formed f r o m decomposition of the tetraalkoxyphosphonium salt ...
... priori, lead to the pentacoordinate phosphorane 1 and (or) the phosphonium salts 2-4 which in turn may lead to either sulfides (collapse of 3) or ethers (collapse of 2 or 4).9 However, we have found that only products which appear to be formed f r o m decomposition of the tetraalkoxyphosphonium salt ...
Making esters from carboxylic acids and alcohols
... Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. The catalyst is usually concentrated sulphuric acid. Dry hydrogen chloride gas is used in some cases, but these tend to involve aromatic esters (ones containing a benzene ring). If you are a UK A ...
... Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. The catalyst is usually concentrated sulphuric acid. Dry hydrogen chloride gas is used in some cases, but these tend to involve aromatic esters (ones containing a benzene ring). If you are a UK A ...
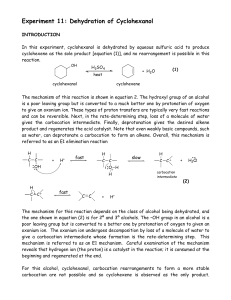
Dehydration of Cyclohexanol
... carbocations derived from certain 2°alcohols may undergo rearrangement to form more stable carbocations. This can result in the formation of rearranged isomeric alkenes. Both 2° and 3° alcohols primarily undergo the E1 reaction under these conditions, whereas for 1° alcohols and methyl alcohol, symm ...
... carbocations derived from certain 2°alcohols may undergo rearrangement to form more stable carbocations. This can result in the formation of rearranged isomeric alkenes. Both 2° and 3° alcohols primarily undergo the E1 reaction under these conditions, whereas for 1° alcohols and methyl alcohol, symm ...
Rhenium(VII) Catalysis of Prins Cyclization Reactions
... more complex aldehyde 17, prepared by a metathesis reaction between crotonaldehyde and the corresponding terminal alkene, was noticeably slower than the others. All of the products showed very good selectivity for the equatorial alcohol THP products. Unsaturated aldehydes will be useful for forming ...
... more complex aldehyde 17, prepared by a metathesis reaction between crotonaldehyde and the corresponding terminal alkene, was noticeably slower than the others. All of the products showed very good selectivity for the equatorial alcohol THP products. Unsaturated aldehydes will be useful for forming ...
PTT102 Aldehydes and Ketones
... 1. Claisen Condensation Condensation of Two Ester Molecules. The product of a Claisen condensation is a βketo ester. In a Claisen condensation, one molecule of carbonyl compound is the nucleophile and second molecule is electrophile. The new C-C bond connect the α-carbon of one molecule and t ...
... 1. Claisen Condensation Condensation of Two Ester Molecules. The product of a Claisen condensation is a βketo ester. In a Claisen condensation, one molecule of carbonyl compound is the nucleophile and second molecule is electrophile. The new C-C bond connect the α-carbon of one molecule and t ...
PTT102 Aldehydes and Ketones
... 1. Claisen Condensation Condensation of Two Ester Molecules. The product of a Claisen condensation is a βketo ester. In a Claisen condensation, one molecule of carbonyl compound is the nucleophile and second molecule is electrophile. The new C-C bond connect the α-carbon of one molecule and t ...
... 1. Claisen Condensation Condensation of Two Ester Molecules. The product of a Claisen condensation is a βketo ester. In a Claisen condensation, one molecule of carbonyl compound is the nucleophile and second molecule is electrophile. The new C-C bond connect the α-carbon of one molecule and t ...
Title Carbonyl reduction with CaH2 and R3SiCl catalyzed by ZnCl2
... high reaction temperature (270 oC). Accordingly, it should be noted that the present hydrosilane formation proceeded under much milder conditions. The results may suggest that the present carbonyl reduction would proceed via hydrosilylation by in situ generated R3SiH.11 However, 1a did not react wit ...
... high reaction temperature (270 oC). Accordingly, it should be noted that the present hydrosilane formation proceeded under much milder conditions. The results may suggest that the present carbonyl reduction would proceed via hydrosilylation by in situ generated R3SiH.11 However, 1a did not react wit ...
Lab 9 - Academic Computer Center
... The overall reduction of a carbonyl group to a hydroxyl group involves the addition of two H atoms. The first H atom comes from a hydride, H-, of NaBH4. The second comes from the workup of the reaction, which is normally conducted in aqueous acid. Sodium borohydride, NaBH4, is the mildest of the th ...
... The overall reduction of a carbonyl group to a hydroxyl group involves the addition of two H atoms. The first H atom comes from a hydride, H-, of NaBH4. The second comes from the workup of the reaction, which is normally conducted in aqueous acid. Sodium borohydride, NaBH4, is the mildest of the th ...
Discussion Sheet 11
... Alcohols can be made into a variety of leaving groups This opens up possibilities for Sn2 and E2 reactions. ...
... Alcohols can be made into a variety of leaving groups This opens up possibilities for Sn2 and E2 reactions. ...
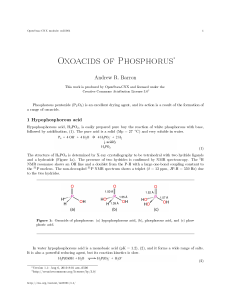
Oxoacids of Phosphorus
... to be comprised of a tetrahedral phosphorus with one hydride and two hydroxides (Figure 1b). 31 P NMR spectroscopy demonstrates the presence of a single hydride by the presence of a doublet as a consequence of the phosphorous center being split by a single hydride (δ = 4 ppm, J P-H = 700 Hz). The 1 ...
... to be comprised of a tetrahedral phosphorus with one hydride and two hydroxides (Figure 1b). 31 P NMR spectroscopy demonstrates the presence of a single hydride by the presence of a doublet as a consequence of the phosphorous center being split by a single hydride (δ = 4 ppm, J P-H = 700 Hz). The 1 ...
Methodology for the olefination of aldehydes and ketones via the Meyer-Schuster reaction
... Phosphorus ylides are prepared before the reaction or in-situ and precautions must be taken due to their sensitivity to moisture and air. The carbanion of the ylide is the characteristic component that allows for nucleophilic attack on the carbonyl carbon. The ylides have been found to demonstrate ...
... Phosphorus ylides are prepared before the reaction or in-situ and precautions must be taken due to their sensitivity to moisture and air. The carbanion of the ylide is the characteristic component that allows for nucleophilic attack on the carbonyl carbon. The ylides have been found to demonstrate ...
19_12_13rw
... This ketone then goes on to react with a R second mole of the Grignard reagent to give a tertiary alcohol. ...
... This ketone then goes on to react with a R second mole of the Grignard reagent to give a tertiary alcohol. ...
CH CH CH CH2 CH3 CH CH3 Br CH CH CH CH2 CH3 CH CH3 F
... e. With salts of carboxylic acids: CH3CH2I + CH3COOK → Reactivity of halogenoalkenes and halogenoarenes Due to a conjugation, i.e. an interaction of lone electron pairs of a halogen atom with the -electrons of either alkenes or arenes, the carbon – halogen bond gets shorter/longer → higher/lower bo ...
... e. With salts of carboxylic acids: CH3CH2I + CH3COOK → Reactivity of halogenoalkenes and halogenoarenes Due to a conjugation, i.e. an interaction of lone electron pairs of a halogen atom with the -electrons of either alkenes or arenes, the carbon – halogen bond gets shorter/longer → higher/lower bo ...
CHAPTER 1: ORGANIC COMPOUNDS
... - C-O-C bond is v-shaped and polar, so molecule is more polar than an alkane with the same number of C’s but not as polar as alcohols with the O-H bond - see table 2 p 46 - can dissolve both polar and non-polar substances - C-O bond stable so they are unrreactive Naming Ethers: - use “-oxy” on end o ...
... - C-O-C bond is v-shaped and polar, so molecule is more polar than an alkane with the same number of C’s but not as polar as alcohols with the O-H bond - see table 2 p 46 - can dissolve both polar and non-polar substances - C-O bond stable so they are unrreactive Naming Ethers: - use “-oxy” on end o ...
Thiobenzoate Photochemistry
... transfer from the amino group in analogy with the reaction of singlet stilbenes. 23 Proton transfer could result in the photoreduction of the thiocarbonyl group. Triphenylamine is a tertiary amine without -C-H bonds. It will reveal what happens in the reaction when the radical is denied a facile a ...
... transfer from the amino group in analogy with the reaction of singlet stilbenes. 23 Proton transfer could result in the photoreduction of the thiocarbonyl group. Triphenylamine is a tertiary amine without -C-H bonds. It will reveal what happens in the reaction when the radical is denied a facile a ...
Exp`t 88 - Chemistry Courses
... Ester syntheses are one of the more enjoyable experiments in organic chemistry, as one can't help but notice the special odors associated with esters - some fruity, some more like nail polish. In contrast, the acids which are used in their syntheses usually have a rotten odor. The reverse reaction, ...
... Ester syntheses are one of the more enjoyable experiments in organic chemistry, as one can't help but notice the special odors associated with esters - some fruity, some more like nail polish. In contrast, the acids which are used in their syntheses usually have a rotten odor. The reverse reaction, ...
The Formation of 2,2,4-Trimethyl-2,3-dihydro-1H-1,5
... Reactions of o-phenylenediamine with a dicarboxylic acid can produce several different products depending on the specific conditions [1]. In the presence of cyclization agents such as hydrochloric acid or polyphosphoric acid, these reactions have been reported to give benzimidazoles [2,3]. This is a ...
... Reactions of o-phenylenediamine with a dicarboxylic acid can produce several different products depending on the specific conditions [1]. In the presence of cyclization agents such as hydrochloric acid or polyphosphoric acid, these reactions have been reported to give benzimidazoles [2,3]. This is a ...
Dehydrating Cyclohexanol
... The results of the Br2 test was that the unknown product changed color which meant that the cyclohexene was truly formed. The results of the (NH4)2Ce(NO3)6 was that the unknown product did turned a little bit red which means there was still some cyclohexanol remained in the product after distillatio ...
... The results of the Br2 test was that the unknown product changed color which meant that the cyclohexene was truly formed. The results of the (NH4)2Ce(NO3)6 was that the unknown product did turned a little bit red which means there was still some cyclohexanol remained in the product after distillatio ...
Discussion Worksheet #10 Formation of Alcohols Skill 1: Functional
... There are ways to control regiochemistry and stereochemistry with alcohol formation ...
... There are ways to control regiochemistry and stereochemistry with alcohol formation ...
Ene reaction

The ene reaction (also known as the Alder-ene reaction) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile), in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the allylic position.This transformation is a group transfer pericyclic reaction, and therefore, usually requires highly activated substrates and/or high temperatures. Nonetheless, the reaction is compatible with a wide variety of functional groups that can be appended to the ene and enophile moieties. Also,many useful Lewis acid-catalyzed ene reactions have been developed which can afford high yields and selectivities at significantly lower temperatures, making the ene reaction a useful C–C forming tool for the synthesis of complex molecules and natural products.