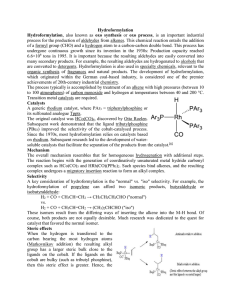
Hydroformylation Hydroformylation, also known as oxo synthesis or
... of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in the 1930s: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary produc ...
... of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in the 1930s: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary produc ...
L-13
... the reaction and led to the production of allylated product 3a in 80% yield (entry 2).[6] Strong Lewis acids such as AlCl3 or BF3・OEt2 were not effective for the allylation (entries 3 and 4), probably because these catalysts are not stable under protic conditions. Sc(OTf)3 only gave a low yield of 3 ...
... the reaction and led to the production of allylated product 3a in 80% yield (entry 2).[6] Strong Lewis acids such as AlCl3 or BF3・OEt2 were not effective for the allylation (entries 3 and 4), probably because these catalysts are not stable under protic conditions. Sc(OTf)3 only gave a low yield of 3 ...
Modules 261 12th edition
... How to test for Chirality: Planes of symmetry Naming Enantiomers: The R, S –System How to Assign (R) and (S) Configurations Properties of Enantiomers: Optical Activity - specific rotation - Plane polarized light - The polarimeter Racemic forms - Racemic forms and Enantiomeric Excess The Synthesis of ...
... How to test for Chirality: Planes of symmetry Naming Enantiomers: The R, S –System How to Assign (R) and (S) Configurations Properties of Enantiomers: Optical Activity - specific rotation - Plane polarized light - The polarimeter Racemic forms - Racemic forms and Enantiomeric Excess The Synthesis of ...
Final Exam Review
... SN2 reaction of an unhindered alkyl halide R-X with OH- to form the alcohol R-OH **Addition of Grignards or R-Li to aldehydes and ketones: mechanism, limitations Reactions of Alcohols: Deprotonation with strong base (NH2-, K metal) to form the alkoxide anion RO– Oxidation to carbonyl - PCC or CrO3 ( ...
... SN2 reaction of an unhindered alkyl halide R-X with OH- to form the alcohol R-OH **Addition of Grignards or R-Li to aldehydes and ketones: mechanism, limitations Reactions of Alcohols: Deprotonation with strong base (NH2-, K metal) to form the alkoxide anion RO– Oxidation to carbonyl - PCC or CrO3 ( ...
Ch 10- Alcohols and Ethers
... – TBDMS groups are stable in a pH range of ~4-12 – They are synthesized by mixing the alcohol with tbutyl chloro dimethyl silane in the presence of an aromatic amine such as: ...
... – TBDMS groups are stable in a pH range of ~4-12 – They are synthesized by mixing the alcohol with tbutyl chloro dimethyl silane in the presence of an aromatic amine such as: ...
FULL PAPER Observations on the Influence of Precursor
... aldehyde 15 by a one-pot reaction via the diol by treatment first with OsO4 / NMO,26 followed by cleavage of the diol with NaIO4.27 A Grignard reaction using vinylmagnesium bromide in THF at –78 °C afforded the racemic mixture of alcohol 16.20 After protection of the hydroxyl group with TIPS to affo ...
... aldehyde 15 by a one-pot reaction via the diol by treatment first with OsO4 / NMO,26 followed by cleavage of the diol with NaIO4.27 A Grignard reaction using vinylmagnesium bromide in THF at –78 °C afforded the racemic mixture of alcohol 16.20 After protection of the hydroxyl group with TIPS to affo ...
Workshop 9
... mechanisms are well established. In other cases they may be speculative and are likely to change as more data become available. Mechanisms map the path by which the reactants change into products and the movement of electrons that accompanies this change. They also show how reactants come together, ...
... mechanisms are well established. In other cases they may be speculative and are likely to change as more data become available. Mechanisms map the path by which the reactants change into products and the movement of electrons that accompanies this change. They also show how reactants come together, ...
PTT102 Aldehydes and Ketones
... The product of a Claisen condensation is a βketo ester. In a Claisen condensation, one molecule of carbonyl compound is the nucleophile and second molecule is electrophile. The new C-C bond connect the α-carbon of one molecule and the carbon that was formerly the carbonyl carbon of the other m ...
... The product of a Claisen condensation is a βketo ester. In a Claisen condensation, one molecule of carbonyl compound is the nucleophile and second molecule is electrophile. The new C-C bond connect the α-carbon of one molecule and the carbon that was formerly the carbonyl carbon of the other m ...
PTT102 Aldehydes and Ketones
... The product of a Claisen condensation is a βketo ester. In a Claisen condensation, one molecule of carbonyl compound is the nucleophile and second molecule is electrophile. The new C-C bond connect the α-carbon of one molecule and the carbon that was formerly the carbonyl carbon of the other m ...
... The product of a Claisen condensation is a βketo ester. In a Claisen condensation, one molecule of carbonyl compound is the nucleophile and second molecule is electrophile. The new C-C bond connect the α-carbon of one molecule and the carbon that was formerly the carbonyl carbon of the other m ...
Review sheet - Paws.wcu.edu.
... Be able to determine a structure, using the 1H NMR spectrum and molecular formula! Reagents for Haloalkanes (X = F, Cl, Br, I) Cl2 or Br2 plus uv light – free radical addition to form a haloalkane SOCl2 and PBr3 (converts R-OH to R-X for 1° and 2° alcohols) HCl / HBr (R-OH to R-X for 3° alcohols) NB ...
... Be able to determine a structure, using the 1H NMR spectrum and molecular formula! Reagents for Haloalkanes (X = F, Cl, Br, I) Cl2 or Br2 plus uv light – free radical addition to form a haloalkane SOCl2 and PBr3 (converts R-OH to R-X for 1° and 2° alcohols) HCl / HBr (R-OH to R-X for 3° alcohols) NB ...
Stereoselective reactions of the carbonyl group
... now finding considerable use as enantioselective catalysts Above is a simple example of epoxide formation The reaction proceeds by SN2 displacement of the bromide by the sulfide and subsequent deprotonation to give the reactive ylide Nucleophilic attack & cyclisation give the epoxide and regenerate ...
... now finding considerable use as enantioselective catalysts Above is a simple example of epoxide formation The reaction proceeds by SN2 displacement of the bromide by the sulfide and subsequent deprotonation to give the reactive ylide Nucleophilic attack & cyclisation give the epoxide and regenerate ...
- EdShare - University of Southampton
... The questions in this resource are based on past exam questions, and are designed to be challenging. The purpose of this resource is as a self-assessment exercise, which you can then look back over using specially made talking mark schemes, explaining the answers to the problems, the process in answ ...
... The questions in this resource are based on past exam questions, and are designed to be challenging. The purpose of this resource is as a self-assessment exercise, which you can then look back over using specially made talking mark schemes, explaining the answers to the problems, the process in answ ...
Organic Chemistry
... Synthesis of Single Enantiomers • We have stressed throughout the text that the synthesis of chiral products from achiral starting materials and under achiral reaction conditions of necessity gives enantiomers as a racemic mixture. • Nature achieves the synthesis of single enantiomers by using enzy ...
... Synthesis of Single Enantiomers • We have stressed throughout the text that the synthesis of chiral products from achiral starting materials and under achiral reaction conditions of necessity gives enantiomers as a racemic mixture. • Nature achieves the synthesis of single enantiomers by using enzy ...
Chapter 7
... • We also saw that the alkynide ion can react with a methyl halide or a primary alkyl halide with no branching at the beta carbon • We should now recognize this as an Sn2 reaction with the alkynide ion as the nucleophile and the alkyl halide as the substrate ...
... • We also saw that the alkynide ion can react with a methyl halide or a primary alkyl halide with no branching at the beta carbon • We should now recognize this as an Sn2 reaction with the alkynide ion as the nucleophile and the alkyl halide as the substrate ...
Chlorotrimethylsilane/Sodium Iodide, a
... Isolated yield. T h e products were characterized by comparing IR, N M R , a n d b p or m p with those of the authentic samples. 10% of N-(1-adamanty1)acetamidewas also isolated in this experiment. c Cholesterol was solubilized using a mixture of chloroform a n d acetonitrile as the sohent. mmol) an ...
... Isolated yield. T h e products were characterized by comparing IR, N M R , a n d b p or m p with those of the authentic samples. 10% of N-(1-adamanty1)acetamidewas also isolated in this experiment. c Cholesterol was solubilized using a mixture of chloroform a n d acetonitrile as the sohent. mmol) an ...
PowerPoint 演示文稿
... the development of new synthetic methods, particularly using chiral catalysts, and the application of computers to synthesis design are among his most notable achievements. Corey has received many honors, including the Wolf Prize (1986), the National Medal of Science (1988), the Japan Prize in Medic ...
... the development of new synthetic methods, particularly using chiral catalysts, and the application of computers to synthesis design are among his most notable achievements. Corey has received many honors, including the Wolf Prize (1986), the National Medal of Science (1988), the Japan Prize in Medic ...
M_ScOrganic_Chemistr..
... amino, carbonyl and carboxyl groups, synthetic strategies for cyclic compounds, retrosynthesis and synthetic approaches to some complex molecules such as camphor, longifoline, prostaglandins etc Unit 2 Carbon-carbon bond forming reactions: Knovenagel condensation, Darzon condensation. Michael additi ...
... amino, carbonyl and carboxyl groups, synthetic strategies for cyclic compounds, retrosynthesis and synthetic approaches to some complex molecules such as camphor, longifoline, prostaglandins etc Unit 2 Carbon-carbon bond forming reactions: Knovenagel condensation, Darzon condensation. Michael additi ...
Lecture 14a - UCLA Chemistry and Biochemistry
... Base: NaOH, KOH, NaOEt (not available), NaH (which cannot be ...
... Base: NaOH, KOH, NaOEt (not available), NaH (which cannot be ...
pcc-sio2.alcohol.oxi..
... Pyridinium chlorochromate (PCC), introduced by Corey and Suggs in 1976 (2), has surpassed the Jones reagent (chromic acid) (3) and the Collins reagent (4) (chromium trioxide/ pyridine complex) in frequency of usage in contemporary organic synthesis. When compared with the Jones reagent, PCC is milde ...
... Pyridinium chlorochromate (PCC), introduced by Corey and Suggs in 1976 (2), has surpassed the Jones reagent (chromic acid) (3) and the Collins reagent (4) (chromium trioxide/ pyridine complex) in frequency of usage in contemporary organic synthesis. When compared with the Jones reagent, PCC is milde ...
Discodermolide

(+)-Discodermolide is a polyketide natural product found to stabilize microtubule. (+)-discodermolide was isolated by Gunasekera and his co-workers at the Harbor Branch Oceanographic Institute from the deep-sea sponge Discodermia dissoluta in 1990. (+)-Discodermolide was found to be a potent inhibitor of tumor cell growth in several MDR cancer cell lines. (+)-discodermolide also shows some unique characters, including a linear backbone structure, immunosuppressive properties both in vitro and in vivo, potent induction of an accelerated senescence phenotype, and synergistic antiproliferative activity in combination with paclitaxel. Discodermolide was recognized as one of the most potent natural promoters of tubulin assembly. A large number of efforts toward the total synthesis of (+)-discodermolide were directed by its interesting biological activities and extreme scarcity of natural sources (0.002% w/w from frozen marine sponge). The compound supply necessary for complete clinical trials cannot be met by harvesting, isolation, and purification. As of 2005, attempts at synthesis or semi-synthesis by fermentation have proven unsuccessful. As a result, all discodermolide used in preclinical studies and clinical trials has come from large-scale total synthesis.























