experiment 10 - Faculty Web Pages
... 2. Assemble a set of chemicals in dropper bottles and obtain a 24-well-plate 3. Each square in the report sheet indicates the two chemicals to be mixed. Place 8 drops of each indicated chemical in the well. Write observations on the report sheet. Note the formation of any precipitate or gas. If ther ...
... 2. Assemble a set of chemicals in dropper bottles and obtain a 24-well-plate 3. Each square in the report sheet indicates the two chemicals to be mixed. Place 8 drops of each indicated chemical in the well. Write observations on the report sheet. Note the formation of any precipitate or gas. If ther ...
CET MODEL QUESTION PAPER 1. Set of quantum numbers (n, /, m
... 12. For an endothermic reaction, where .:\11 represents the enthalpy of the reaction in KJ / mole, the minimum value for the energy of activation will be 1] less than ∆H 2] zero 3] more than ∆H 4] equal to ∆H 13. Zone refining is a method to obtain I] very high temperature 2] ultra pure Al 14. An or ...
... 12. For an endothermic reaction, where .:\11 represents the enthalpy of the reaction in KJ / mole, the minimum value for the energy of activation will be 1] less than ∆H 2] zero 3] more than ∆H 4] equal to ∆H 13. Zone refining is a method to obtain I] very high temperature 2] ultra pure Al 14. An or ...
Chem 30BL_Lecture 2_.. - UCLA Chemistry and Biochemistry
... enthalpy (DH=23.9 kJ, ) nor the entropy (DS=84.91 J, ) changes much in the reaction and they also display opposing trends. Thus, the equilibrium constant is Keq=1.8 at 25 oC and Keq=8 at 80 oC, which are both low. ...
... enthalpy (DH=23.9 kJ, ) nor the entropy (DS=84.91 J, ) changes much in the reaction and they also display opposing trends. Thus, the equilibrium constant is Keq=1.8 at 25 oC and Keq=8 at 80 oC, which are both low. ...
Chem 30BL * Lecture 2 - UCLA Chemistry and Biochemistry
... enthalpy (DH=23.9 kJ, ) nor the entropy (DS=84.91 J, ) changes much in the reaction and they also display opposing trends. Thus, the equilibrium constant is Keq=1.8 at 25 oC and Keq=8 at 80 oC, which are both low. ...
... enthalpy (DH=23.9 kJ, ) nor the entropy (DS=84.91 J, ) changes much in the reaction and they also display opposing trends. Thus, the equilibrium constant is Keq=1.8 at 25 oC and Keq=8 at 80 oC, which are both low. ...
+ Y
... has an electron-poor atom (e.g H+, CH3+ ) and can form a bond by accepting a pair of electrons from a nucleophile ...
... has an electron-poor atom (e.g H+, CH3+ ) and can form a bond by accepting a pair of electrons from a nucleophile ...
Grignard Reaction - This is Synthesis
... Heating beyond the boiling point of the employed solvent allows reduction of the reaction time. Typically the formation of the Grignard reagent as well as the Grignard reaction itself require 30-60 minutes at reflux. Under sealed vessel conditions both steps, especially the Grignard reaction, can be ...
... Heating beyond the boiling point of the employed solvent allows reduction of the reaction time. Typically the formation of the Grignard reagent as well as the Grignard reaction itself require 30-60 minutes at reflux. Under sealed vessel conditions both steps, especially the Grignard reaction, can be ...
Problem Set: Empirical and Molecular Formulas
... 4. Using the same reaction for the production of ammonia as in #3, determine the percent yield when 400.0 kg of H2 are added to an excess of N2, and 1040. kg of NH3 are produced. ...
... 4. Using the same reaction for the production of ammonia as in #3, determine the percent yield when 400.0 kg of H2 are added to an excess of N2, and 1040. kg of NH3 are produced. ...
Nucleophilic Substitution Reaction
... In above mechanism the overall rate is limited to that of the slower second stage which depends only on the concentration of the conjugate base of the reactant. This mechanism is called as E1cB which means elimination, unimolecular, conjugate base. The distinction between E2 and E1cB mechanism can b ...
... In above mechanism the overall rate is limited to that of the slower second stage which depends only on the concentration of the conjugate base of the reactant. This mechanism is called as E1cB which means elimination, unimolecular, conjugate base. The distinction between E2 and E1cB mechanism can b ...
OrganicChem10 RxPaths SOLUTIONS (2014)
... E. ethanoic acid (from D) + ethanamine (from B) to N-ethylethanamide (condensation) CH3COOH + H2NCH2CH3 CH3CONCH2CH3 + H2O ...
... E. ethanoic acid (from D) + ethanamine (from B) to N-ethylethanamide (condensation) CH3COOH + H2NCH2CH3 CH3CONCH2CH3 + H2O ...
Exam 2 SOLUTION
... a) How many steps are in the mechanism to this reaction? There are 4 steps to this mechanism. b) Which step is the rate-determining step? The second step, with the highest activation barrier, is the RDS. c) Label ∆Hrxn and the activation energies on the graph. 4. Using curved-arrow notation, give th ...
... a) How many steps are in the mechanism to this reaction? There are 4 steps to this mechanism. b) Which step is the rate-determining step? The second step, with the highest activation barrier, is the RDS. c) Label ∆Hrxn and the activation energies on the graph. 4. Using curved-arrow notation, give th ...
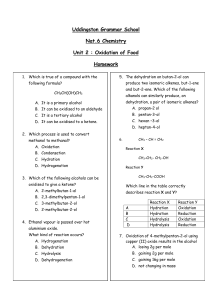
2d Oxidation of Food Homework
... In the reaction of propanal with Tollens’ reagent, silver ions are reduced to form silver metal. Complete the following ion-electron equation for the oxidation. C3H6O ...
... In the reaction of propanal with Tollens’ reagent, silver ions are reduced to form silver metal. Complete the following ion-electron equation for the oxidation. C3H6O ...
Named Reactions Of Haloalkanes and haloarenes
... 4)Swarts reaction– The synthesis of alkyl fluoride is best accomplished by heating n alkyl chloride in the presence of metallic fluoride such as AgF,Hg2F2,CoF2 ...
... 4)Swarts reaction– The synthesis of alkyl fluoride is best accomplished by heating n alkyl chloride in the presence of metallic fluoride such as AgF,Hg2F2,CoF2 ...
Assignment 4 Task 1a
... have been assigned to a new case and are working as part of a team to solve the case. Working in the laboratory you will need to have a good understanding of the conventions adopted to ensure that all chemical compounds have unambiguous names. You also need to understand how a combination of element ...
... have been assigned to a new case and are working as part of a team to solve the case. Working in the laboratory you will need to have a good understanding of the conventions adopted to ensure that all chemical compounds have unambiguous names. You also need to understand how a combination of element ...
Step 1
... QUESTION – Which is the stronger base a primary amine or secondary amine? Secondary amines are stronger bases than primary amines because they have more alkyl groups that are substituted onto the N atom in place of H atoms. Therefore more electrons are pushed onto the N atom (because of the pos ...
... QUESTION – Which is the stronger base a primary amine or secondary amine? Secondary amines are stronger bases than primary amines because they have more alkyl groups that are substituted onto the N atom in place of H atoms. Therefore more electrons are pushed onto the N atom (because of the pos ...
Eliminations
... SN1/SN2/E2/E1 summary/comparison: (1) Primary alkyl halides will prefer SN2 unless a strong hindered base is used in which case E2 will be favored. For example, t-‐butoxide is a sterically hindered base. ...
... SN1/SN2/E2/E1 summary/comparison: (1) Primary alkyl halides will prefer SN2 unless a strong hindered base is used in which case E2 will be favored. For example, t-‐butoxide is a sterically hindered base. ...
Formative 3.5 2014
... Any ester with 6 carbons. Any 6 carbon carboxylic acid. (c) (i) Butan-1-ol reacts with concentrated H2SO4 to form but-1-ene and water. This is an elimination reaction. Since this is a primary alcohol with the –OH group at the end of the carbon chain there is only one possible product. Butan-2-ol is ...
... Any ester with 6 carbons. Any 6 carbon carboxylic acid. (c) (i) Butan-1-ol reacts with concentrated H2SO4 to form but-1-ene and water. This is an elimination reaction. Since this is a primary alcohol with the –OH group at the end of the carbon chain there is only one possible product. Butan-2-ol is ...
Total marks available
... This is a question about halogenoalkanes. (a) Halogenoalkanes can react with hydroxide ions in different ways depending on the conditions used. Using 1-chloro-1-fluoroethane, CH3CHClF, as an example of a halogenoalkane, the following reaction could occur in aqueous solution. CH3CHClF + OH− → CH3CHOH ...
... This is a question about halogenoalkanes. (a) Halogenoalkanes can react with hydroxide ions in different ways depending on the conditions used. Using 1-chloro-1-fluoroethane, CH3CHClF, as an example of a halogenoalkane, the following reaction could occur in aqueous solution. CH3CHClF + OH− → CH3CHOH ...
Document
... The highly reactive species methylene, H2C: (the simplest carbene) can be produced from the decomposition of diazomethane: ...
... The highly reactive species methylene, H2C: (the simplest carbene) can be produced from the decomposition of diazomethane: ...
Chapter 9. Addition Reactions of Alkenes
... The reaction below, which provides compound M as its major product, appears to defy the principles that we discussed in class. Draw the structures of the intermediate carbocations that form in this reaction, then clearly but briefly explain why M, and not L, is the major product of this reaction. Hi ...
... The reaction below, which provides compound M as its major product, appears to defy the principles that we discussed in class. Draw the structures of the intermediate carbocations that form in this reaction, then clearly but briefly explain why M, and not L, is the major product of this reaction. Hi ...
Octenes from E1 versus E2 Eliminations
... Fill a 10 x 100 mm reaction tube to the 0.5 mL mark with 1-octanol (n-octyl alcohol) and insert a 1/2-inch stir bar. Add 5 drops of conc. sulfuric acid. While stirring, heat the reaction for 20 to 30 minutes. At first you will see water droplets and a cloudy liquid condensing on the walls of the rea ...
... Fill a 10 x 100 mm reaction tube to the 0.5 mL mark with 1-octanol (n-octyl alcohol) and insert a 1/2-inch stir bar. Add 5 drops of conc. sulfuric acid. While stirring, heat the reaction for 20 to 30 minutes. At first you will see water droplets and a cloudy liquid condensing on the walls of the rea ...
Organic Reactions Worksheet
... For the questions below, give full structural diagrams and names for all reactants and products. Also indicate any catalysts/reaction conditions for the reaction. Also indicate the TYPE OF REACTION. 1. Oxidation with primary and secondary alcohols via sulfuric acid and potassium dichromate. a. What ...
... For the questions below, give full structural diagrams and names for all reactants and products. Also indicate any catalysts/reaction conditions for the reaction. Also indicate the TYPE OF REACTION. 1. Oxidation with primary and secondary alcohols via sulfuric acid and potassium dichromate. a. What ...
Organic Reactions 2.1- 2.3 - mccormack-sch4u-2013
... water adds to a bond splitting it into two Reverse of a condensation reaction Water can add to an ester or amide bond Ester + water makes a carboxylic acid and alcohol Amide + water makes a carboxylic acid and amine ...
... water adds to a bond splitting it into two Reverse of a condensation reaction Water can add to an ester or amide bond Ester + water makes a carboxylic acid and alcohol Amide + water makes a carboxylic acid and amine ...
National 5 Whole Course Revision Questions Unit 1 Chemical
... chemical reaction- mention collision theory in your answer. 2. a) How do catalysts affect the rate of a chemical reaction? b) Name the types of catalysts and describe how they differ from each other? 3. What is an enzyme and state the use of one. 4. Calculate the rate of reaction if 20cm3 of carbon ...
... chemical reaction- mention collision theory in your answer. 2. a) How do catalysts affect the rate of a chemical reaction? b) Name the types of catalysts and describe how they differ from each other? 3. What is an enzyme and state the use of one. 4. Calculate the rate of reaction if 20cm3 of carbon ...
File
... 1. Name the type of reaction and draw structural diagrams to represent the following reactions: a) Reaction of propene to form an alcohol. b) Reaction of 3-methyl-2-pentanol with HBr. c) Reaction of 1-bromo-3-methylpropane with sodium hydroxide. 2. Write balanced equations and name the reactants and ...
... 1. Name the type of reaction and draw structural diagrams to represent the following reactions: a) Reaction of propene to form an alcohol. b) Reaction of 3-methyl-2-pentanol with HBr. c) Reaction of 1-bromo-3-methylpropane with sodium hydroxide. 2. Write balanced equations and name the reactants and ...
Hofmann–Löffler reaction

The Hofmann–Löffler reaction (also referred to as Hofmann–Löffler–Freytag reaction, Löffler–Freytag reaction, Löffler–Hofmann reaction, as well as Löffler's method) is an organic reaction in which a cyclic amine 2 (pyrrolidine or, in some cases, piperidine) is generated by thermal or photochemical decomposition of N-halogenated amine 1 in the presence of a strong acid (concentrated sulfuric acid or concentrated CF3CO2H). The Hofmann–Löffler–Freytag reaction proceeds via an intramolecular hydrogen atom transfer to a nitrogen-centered radical and is an example of a remote intramolecular free radical C–H functionalization.