1. Four of the structural isomers of C4H10O are alcohols. One of
... Calculate the maximum possible mass of butan-2-ol which could be obtained in the above experiment. ...
... Calculate the maximum possible mass of butan-2-ol which could be obtained in the above experiment. ...
Rapid, Controlled Assembly of Polyenes for Studying Pericyclic
... David A. Vosburg, Department of Chemistry, Harvey Mudd College Pericyclic reactions are among the most powerful transformations in organic chemistry, and they are even more impressive when they occur in tandem. Outstanding examples of pericyclic reaction cascades are found in the biosyntheses of the ...
... David A. Vosburg, Department of Chemistry, Harvey Mudd College Pericyclic reactions are among the most powerful transformations in organic chemistry, and they are even more impressive when they occur in tandem. Outstanding examples of pericyclic reaction cascades are found in the biosyntheses of the ...
Slide 1
... *To show the equation & the mechanism of the reaction. *To gain some knowledge about the applications & recent literatures related to the reaction. ...
... *To show the equation & the mechanism of the reaction. *To gain some knowledge about the applications & recent literatures related to the reaction. ...
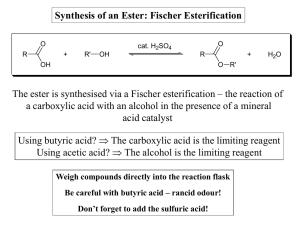
Synthesis of an Ester: Fischer Esterification The ester is synthesised
... - Removing one of the products (ester or water) from the reaction, as it is formed. - Using an even larger excess of one of the reagents. - Employing a carboxylic acid derivative, such as an acid chloride, which makes the reaction irreversible. ...
... - Removing one of the products (ester or water) from the reaction, as it is formed. - Using an even larger excess of one of the reagents. - Employing a carboxylic acid derivative, such as an acid chloride, which makes the reaction irreversible. ...
Taylor`s Organic Reactions Summary Sheet
... Alcohols are formed by addition reactions between an Alkene/Alkyne and water this is called a hydration reaction. The hydrogen attaches to the carbon atom that already has more hydrogen atoms; the –OH group attaches to the other carbon atom in the double bond. Hydration Reaction: A reaction that res ...
... Alcohols are formed by addition reactions between an Alkene/Alkyne and water this is called a hydration reaction. The hydrogen attaches to the carbon atom that already has more hydrogen atoms; the –OH group attaches to the other carbon atom in the double bond. Hydration Reaction: A reaction that res ...
3.4 How do we use the Activity Series
... 3. Will the above reaction take place?_____________________________________________________ 4. Will the reverse reaction take place, Cl2 + 2NaF? __________________________________________ ...
... 3. Will the above reaction take place?_____________________________________________________ 4. Will the reverse reaction take place, Cl2 + 2NaF? __________________________________________ ...
CLASS-X SC (Chemical Reactions and Equations)
... (a) MgSO4 (b) Na2 CO3 (c) NaHCO3 (d) MgCO3 6. The H+ ion concentration of a solution is1.0×10−5m. The solution is (a) Acidic (b) Alkaline (c) Neutral (d) Amphoteric 7. An aqueous solution with pH-zero is (a) Acidic (b) Alkaline (c) Neutral (d) Amphoteric 8. Setting of Plaster of Paris takes place du ...
... (a) MgSO4 (b) Na2 CO3 (c) NaHCO3 (d) MgCO3 6. The H+ ion concentration of a solution is1.0×10−5m. The solution is (a) Acidic (b) Alkaline (c) Neutral (d) Amphoteric 7. An aqueous solution with pH-zero is (a) Acidic (b) Alkaline (c) Neutral (d) Amphoteric 8. Setting of Plaster of Paris takes place du ...
org test 1
... VBPS CLASS: XII SUBJECT: CHEMISTRY ASSIGNMENT TYPE: REVISION OF UNITS 11-14 1. Why alkyl halides are generally not prepared in laboratory by free radical halogenation of alkanes? 2. Why is Sulphuric acid not used during reaction of alcohol with KI? 3. Why is preparation of ethers by acid catalysed d ...
... VBPS CLASS: XII SUBJECT: CHEMISTRY ASSIGNMENT TYPE: REVISION OF UNITS 11-14 1. Why alkyl halides are generally not prepared in laboratory by free radical halogenation of alkanes? 2. Why is Sulphuric acid not used during reaction of alcohol with KI? 3. Why is preparation of ethers by acid catalysed d ...
Chemistry Final Test
... the temperature within the range of the original measurements e Arrhenius equation can not be applied to aqueous phase reaction f reactions with higher Ea have rates that depend more strongly on the temperature ...
... the temperature within the range of the original measurements e Arrhenius equation can not be applied to aqueous phase reaction f reactions with higher Ea have rates that depend more strongly on the temperature ...
COUPLING REACTIONS IN ORGANIC SYNTHESIS
... The addition of dihydrogen to Vaska's complex and other transition metals is a reversible reaction. The hydrogen can be released again if the reaction moves to the left in a reductive elimination. That reversibility makes transition metal compounds useful for hydrogen storage. Hydrogen gas is volumi ...
... The addition of dihydrogen to Vaska's complex and other transition metals is a reversible reaction. The hydrogen can be released again if the reaction moves to the left in a reductive elimination. That reversibility makes transition metal compounds useful for hydrogen storage. Hydrogen gas is volumi ...
Chapter 20 Amines-part 2
... Sandmeyer Reaction: Replacement of Diazonium Ion by Cl, Br or CN t Mechanism of the Sandmeyer reaction is not well -understood but is thought to occur via radicals ...
... Sandmeyer Reaction: Replacement of Diazonium Ion by Cl, Br or CN t Mechanism of the Sandmeyer reaction is not well -understood but is thought to occur via radicals ...
Q 1: Molecular formula of BHA is
... Q 23: Oxidation product of glucose with Br2 water is a gluconic acid b saccharic acid ...
... Q 23: Oxidation product of glucose with Br2 water is a gluconic acid b saccharic acid ...
Preface - Wiley Online Library
... atom. Nitrogen boasts the strongest homoatomic bond rendering it chemically rather inert, but yet its fixation into plant material can be accomplished under the mildest of conditions. In his marvelous book, The Disappearing Spoon, Sean McKean eloquently summed up the idiosyncratic character of nitro ...
... atom. Nitrogen boasts the strongest homoatomic bond rendering it chemically rather inert, but yet its fixation into plant material can be accomplished under the mildest of conditions. In his marvelous book, The Disappearing Spoon, Sean McKean eloquently summed up the idiosyncratic character of nitro ...
chemistry pretest - the Biology Scholars Program Wiki
... taken these courses, I do not expect you to be up to speed with all of these questions. However, it is extremely important that you become comfortable with them as soon as possible and I would like to help you with that before the new content kicks in full speed. Therefore, I would like you to work ...
... taken these courses, I do not expect you to be up to speed with all of these questions. However, it is extremely important that you become comfortable with them as soon as possible and I would like to help you with that before the new content kicks in full speed. Therefore, I would like you to work ...
C h e m g u i d e ... ALCOHOLS: ESTERIFICATION
... which is about esterification using acyl chlorides. Worry about this when the topic comes up later in your course. ...
... which is about esterification using acyl chlorides. Worry about this when the topic comes up later in your course. ...
Slide 1
... The equilibrium constant for the reaction depends on the relative stabilities of the reactants and products ...
... The equilibrium constant for the reaction depends on the relative stabilities of the reactants and products ...
Exam 2 Review A
... three things: 1. React with nucleophiles, 2. -eliminate (lose H+) to form an alkene, 3. undergo skeletal rearrangements via 1,2-hydride shifts or 1,2-methanide shifts. [we will defer discussion of #3 until Chapter 7]. Remember, carbocation stability plays a role in analyzing transition states, whic ...
... three things: 1. React with nucleophiles, 2. -eliminate (lose H+) to form an alkene, 3. undergo skeletal rearrangements via 1,2-hydride shifts or 1,2-methanide shifts. [we will defer discussion of #3 until Chapter 7]. Remember, carbocation stability plays a role in analyzing transition states, whic ...
Exam 2 Review A
... three things: 1. React with nucleophiles, 2. -eliminate (lose H+) to form an alkene, 3. undergo skeletal rearrangements via 1,2-hydride shifts or 1,2-methanide shifts. [we will defer discussion of #3 until Chapter 7]. Remember, carbocation stability plays a role in analyzing transition states, whic ...
... three things: 1. React with nucleophiles, 2. -eliminate (lose H+) to form an alkene, 3. undergo skeletal rearrangements via 1,2-hydride shifts or 1,2-methanide shifts. [we will defer discussion of #3 until Chapter 7]. Remember, carbocation stability plays a role in analyzing transition states, whic ...
CH 115 Exam 2 - UAB General Chemistry Supplemental Instruction
... Assume the chemical equations on this exam are NOT balanced unless stated otherwise. 1. Balance the equation and give the stoichiometric coefficient for HCl ...
... Assume the chemical equations on this exam are NOT balanced unless stated otherwise. 1. Balance the equation and give the stoichiometric coefficient for HCl ...
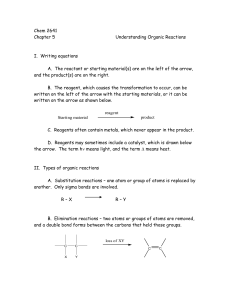
Chem 2641 Chapter 5 Understanding Organic Reactions I. Writing
... reaction is called the mechanism of the reaction. B. Reactions can be one step or more than one step. 1. Reactions that are one step are called concerted reactions. The reactant is converted directly to product. 2. Reactions that require more than one step contain species called intermediates. These ...
... reaction is called the mechanism of the reaction. B. Reactions can be one step or more than one step. 1. Reactions that are one step are called concerted reactions. The reactant is converted directly to product. 2. Reactions that require more than one step contain species called intermediates. These ...
lec-2- 211(ES +Add)
... Benzene is treated with a mixture of concentrated nitric acid and concentrated sulphuric acid at a temperature not exceeding 50°C. As temperature increases there is a greater chance of getting more than one nitro group, -NO2, substituted onto the ring. Nitrobenzene is formed. H2SO4 ...
... Benzene is treated with a mixture of concentrated nitric acid and concentrated sulphuric acid at a temperature not exceeding 50°C. As temperature increases there is a greater chance of getting more than one nitro group, -NO2, substituted onto the ring. Nitrobenzene is formed. H2SO4 ...
Hofmann–Löffler reaction

The Hofmann–Löffler reaction (also referred to as Hofmann–Löffler–Freytag reaction, Löffler–Freytag reaction, Löffler–Hofmann reaction, as well as Löffler's method) is an organic reaction in which a cyclic amine 2 (pyrrolidine or, in some cases, piperidine) is generated by thermal or photochemical decomposition of N-halogenated amine 1 in the presence of a strong acid (concentrated sulfuric acid or concentrated CF3CO2H). The Hofmann–Löffler–Freytag reaction proceeds via an intramolecular hydrogen atom transfer to a nitrogen-centered radical and is an example of a remote intramolecular free radical C–H functionalization.