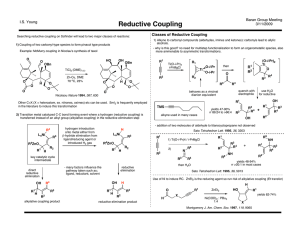
Direct conversion of cellulose into sorbitol using dual
... respectively. Liquid-phase mineral acids or heteropolyacids have been used together with metal catalysts containing Pt or Ru [8,9]. These acids, however, are difficult to recover and can cause corrosion of the reactor. In addition, a large amount of waste sludge is formed in the acid neutralization p ...
... respectively. Liquid-phase mineral acids or heteropolyacids have been used together with metal catalysts containing Pt or Ru [8,9]. These acids, however, are difficult to recover and can cause corrosion of the reactor. In addition, a large amount of waste sludge is formed in the acid neutralization p ...
Heterogeneous Catalysts for Biodiesel Production
... of a solid catalyst in biodiesel production could reduce its price, so biodiesel could become competitive with diesel also from an economic point of view. Therefore, great research efforts have been underway recently to find the right catalysts. Several general reviews on biofuels and biodiesel prod ...
... of a solid catalyst in biodiesel production could reduce its price, so biodiesel could become competitive with diesel also from an economic point of view. Therefore, great research efforts have been underway recently to find the right catalysts. Several general reviews on biofuels and biodiesel prod ...
updated chem cp final review key
... 43. Know what conditions are true of a chemical reaction at equilibrium. (1) rates of forward and reverse reactions are equal. (2) The concentrations of all substances involved stop changing (3) reactants and products are both present but concentrations are not necessarily equal (4) The system is a ...
... 43. Know what conditions are true of a chemical reaction at equilibrium. (1) rates of forward and reverse reactions are equal. (2) The concentrations of all substances involved stop changing (3) reactants and products are both present but concentrations are not necessarily equal (4) The system is a ...
6.10 Acid-Catalyzed Hydration of Alkenes
... Hydroboration can be viewed as the addition of borane (BH3) to the double bond. But BH3 is not the reagent actually used. ...
... Hydroboration can be viewed as the addition of borane (BH3) to the double bond. But BH3 is not the reagent actually used. ...
57 estonian national chemistry olympiad
... of B can be written as AX3. If gas B is heated in hydrogen atmosphere, elementary compound A If formed. A is also formed by thermal decomposition of iodide AI3 and in reaction of oxide A2O3 with magnesium. The second product in three given reactions of A formation are strong acid C, elementary subst ...
... of B can be written as AX3. If gas B is heated in hydrogen atmosphere, elementary compound A If formed. A is also formed by thermal decomposition of iodide AI3 and in reaction of oxide A2O3 with magnesium. The second product in three given reactions of A formation are strong acid C, elementary subst ...
Document
... participates in several types of enzymecatalyzed oxidation/reduction reactions • our concern is its participation in the oxidation of a CC bond in a fatty acid hydrocarbon chain to a C=C bond as shown in these balanced half-reactions Oxidation of the hydrocarbon chain: - CH 2 - CH2 - CH = CH - + 2 H ...
... participates in several types of enzymecatalyzed oxidation/reduction reactions • our concern is its participation in the oxidation of a CC bond in a fatty acid hydrocarbon chain to a C=C bond as shown in these balanced half-reactions Oxidation of the hydrocarbon chain: - CH 2 - CH2 - CH = CH - + 2 H ...
One-Pot Catalytic Conversion of Cellulose and of Woody
... 15/60/25 Cu/Mg/Al molar ratio and was prepared by calcining a copper-doped hydrotalcite as previously reported.12,13 Small-Scale Batch Reactions. In a typical run, a finely divided substrate (wood or cellulose), catalyst (usually Cu20-PMO), and methanol (3.0 mL) were added to a 10 mL stainless steel ...
... 15/60/25 Cu/Mg/Al molar ratio and was prepared by calcining a copper-doped hydrotalcite as previously reported.12,13 Small-Scale Batch Reactions. In a typical run, a finely divided substrate (wood or cellulose), catalyst (usually Cu20-PMO), and methanol (3.0 mL) were added to a 10 mL stainless steel ...
06. Alcohols. Phenols. Ethers
... are called monohydric alcohols. These are further classified as primary (1'), secondary (2'), and tertiary (3') according as the ОН group is attached to primary, secondary and tertiary carbon atoms respectively. For example: ...
... are called monohydric alcohols. These are further classified as primary (1'), secondary (2'), and tertiary (3') according as the ОН group is attached to primary, secondary and tertiary carbon atoms respectively. For example: ...
Problem 28. TUNNELING IN CHEMISTRY
... the HCl molecule the chemical bond is 17% ionic in character. Find the absolute values of ccov and cion for HCl. ...
... the HCl molecule the chemical bond is 17% ionic in character. Find the absolute values of ccov and cion for HCl. ...
Chapter 3 Mass Relationships in Chemical Reactions 1
... 103. One way of obtaining pure sodium carbonate is through the decomposition of the mineral trona, Na5(CO3)2(HCO3)·2H2O, Na5(CO3)2(HCO3)·2H2O(s) → 5Na2CO3(s) + CO2(g) + 3H2O(g) When 1.00 metric ton (1 × 103 kg) of trona is decomposed, 0.74 metric ton of Na2CO3 is recovered. What is the percent yield ...
... 103. One way of obtaining pure sodium carbonate is through the decomposition of the mineral trona, Na5(CO3)2(HCO3)·2H2O, Na5(CO3)2(HCO3)·2H2O(s) → 5Na2CO3(s) + CO2(g) + 3H2O(g) When 1.00 metric ton (1 × 103 kg) of trona is decomposed, 0.74 metric ton of Na2CO3 is recovered. What is the percent yield ...
Precision, accuracy and significant figures
... Phosphoric acid can be generated by the oxidation of phosphorus with nitric acid according to the following equation: P(s) + 5HNO3(aq) → H3PO4(aq) + H2O(l) + 5NO2(g) If sufficient reactants are available to produce 1.00 kg of phosphoric acid (H3PO4), what mass of nitrogen dioxide will also be genera ...
... Phosphoric acid can be generated by the oxidation of phosphorus with nitric acid according to the following equation: P(s) + 5HNO3(aq) → H3PO4(aq) + H2O(l) + 5NO2(g) If sufficient reactants are available to produce 1.00 kg of phosphoric acid (H3PO4), what mass of nitrogen dioxide will also be genera ...
1 -
... IUPAC nomenclature (1) Parent name is alkyne i.e. change the -ane to -yne. Choose the longest continuous chain containing the triple bond as the basis for the parent name. The position of the triple bond takes the lowest possible number. If both double and triple bonds are present, the e ...
... IUPAC nomenclature (1) Parent name is alkyne i.e. change the -ane to -yne. Choose the longest continuous chain containing the triple bond as the basis for the parent name. The position of the triple bond takes the lowest possible number. If both double and triple bonds are present, the e ...
Nuclear Debate - Noadswood Science
... of Ethene Ethanol can be produced in 3 major ways. You need to know the advantages and disadvantages of each method Ethene, which is obtained from crude oil, can be reacted with steam under high temperature and pressure to form pure ethanol. ...
... of Ethene Ethanol can be produced in 3 major ways. You need to know the advantages and disadvantages of each method Ethene, which is obtained from crude oil, can be reacted with steam under high temperature and pressure to form pure ethanol. ...
Chapter 4: Reactions in Aqueous Solution
... # moles NaOH = 0.288 moles NaOH Therefore, M = 0.288 mol NaOH / 1.50 L = 0.192 M (Reasonable) ________________________________________________________________ 5) Diluting Concentrated Solutions A) Dilution - process by which water (or another solvent) is added to a solution of known concentration to ...
... # moles NaOH = 0.288 moles NaOH Therefore, M = 0.288 mol NaOH / 1.50 L = 0.192 M (Reasonable) ________________________________________________________________ 5) Diluting Concentrated Solutions A) Dilution - process by which water (or another solvent) is added to a solution of known concentration to ...
Principles of Chemistry 1 and 2 Notes
... Partial charge confirmed by electrical field (turning it on/off) Generally speaking: Compounds that possess dipole moments are said to be polar and compounds that do not possess dipole moments are said to be non polar. Examples: Diatomic molecules: CO, NO, HCl, HF, etc…. all have dipole moments and ...
... Partial charge confirmed by electrical field (turning it on/off) Generally speaking: Compounds that possess dipole moments are said to be polar and compounds that do not possess dipole moments are said to be non polar. Examples: Diatomic molecules: CO, NO, HCl, HF, etc…. all have dipole moments and ...
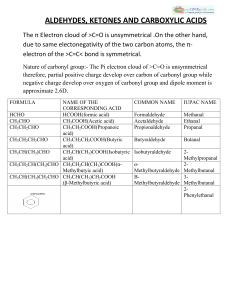
ALDEHYDES, KETONES AND CARBOXYLIC ACIDS
... :-Lower members are soluble in water because they can form H-bond with water. :-Higher members are insoluble in water due to large size of their hydrophobic group. :-Aldehydes are prepared bya. Dehydrogenation of primary alcohols b. Controlled oxidation of primary alcohols. c. Controlled and selecti ...
... :-Lower members are soluble in water because they can form H-bond with water. :-Higher members are insoluble in water due to large size of their hydrophobic group. :-Aldehydes are prepared bya. Dehydrogenation of primary alcohols b. Controlled oxidation of primary alcohols. c. Controlled and selecti ...
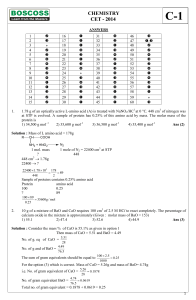
CHEMISTRY CET
... 'Z' is slowly passed into an aqueous solution of Y, colloidal sulphur is obtained. X and Z could be, respectively 1) Na2SO4, H2S 2) Na2SO4, SO2 3) Na2S, SO3 4) Na2SO3, H2S Ans (4) ...
... 'Z' is slowly passed into an aqueous solution of Y, colloidal sulphur is obtained. X and Z could be, respectively 1) Na2SO4, H2S 2) Na2SO4, SO2 3) Na2S, SO3 4) Na2SO3, H2S Ans (4) ...
Chapter 11 - Department of Chemistry and Physics
... methylene group. The boiling points of the alkanes increase with molecular weight. All alkanes have this general formula. ...
... methylene group. The boiling points of the alkanes increase with molecular weight. All alkanes have this general formula. ...
Molecular orbital approach to substituent effects in amine
... Amine-C02 Interactions a fixed value of E”), as long as E , < E,, the higher the energy of the HOMO, the greater the importance of the orbital-control term. Similarly, the lower the charge a t the donor site, the less important the charge-control term. These and other arguments18J2 show that a donor ...
... Amine-C02 Interactions a fixed value of E”), as long as E , < E,, the higher the energy of the HOMO, the greater the importance of the orbital-control term. Similarly, the lower the charge a t the donor site, the less important the charge-control term. These and other arguments18J2 show that a donor ...
p-BLOCK ELEMENTS - einstein classes
... An alternative test is to make the ester methyl borate B(OCH3)3. The suspected borate sample is mixed with concentrated H2SO4 to form H3BO3, and warmed with methyl alcohol in a small evaporating basin. B(OH)3 + 3CH3OH B(OCH3)3 + 3H2O The concentrated H2SO4 removes the water formed. The mixture is ...
... An alternative test is to make the ester methyl borate B(OCH3)3. The suspected borate sample is mixed with concentrated H2SO4 to form H3BO3, and warmed with methyl alcohol in a small evaporating basin. B(OH)3 + 3CH3OH B(OCH3)3 + 3H2O The concentrated H2SO4 removes the water formed. The mixture is ...
19_Worked_Examples
... metal to the cooler water. The final temperature, after the metal and water achieve the same temperature (thermal equilibrium), will be somewhere between the initial temperatures of the metal and the water. (b) Experience tells us that this process is not spontaneous— we certainly have never seen hy ...
... metal to the cooler water. The final temperature, after the metal and water achieve the same temperature (thermal equilibrium), will be somewhere between the initial temperatures of the metal and the water. (b) Experience tells us that this process is not spontaneous— we certainly have never seen hy ...
Mechanistic and Computational Studies of Ferroin, Simple Organic
... Before the year 1950, many chemists in their ‘right mind’ held the archaic belief that all chemical reactions proceed strictly from reactants to products, though some could be coaxed into reverse. What we now know colloquially as a potential energy surface was only visualized in more than two dimens ...
... Before the year 1950, many chemists in their ‘right mind’ held the archaic belief that all chemical reactions proceed strictly from reactants to products, though some could be coaxed into reverse. What we now know colloquially as a potential energy surface was only visualized in more than two dimens ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.