PDF - Nanyang Technological University
... iminiums or their analogues.[4d] However, this approach with chiral ligands was found to be unsuccessful for reactions starting with simple ketone nucleophiles, as reported by Klussmann and co-workers[11a] as well as Xie and Huang.[4c] Approaches that use asymmetric enamine catalysis[13] for the act ...
... iminiums or their analogues.[4d] However, this approach with chiral ligands was found to be unsuccessful for reactions starting with simple ketone nucleophiles, as reported by Klussmann and co-workers[11a] as well as Xie and Huang.[4c] Approaches that use asymmetric enamine catalysis[13] for the act ...
Name
... the saturated solution pictured here: A. The total amount of dissolved solute remains constant. B. The total mass of undissolved crystals remains constant. C. When the rate of solvation equals the rate of crystallization, a state of dynamic equilibrium exists. D. If more solute were added to the con ...
... the saturated solution pictured here: A. The total amount of dissolved solute remains constant. B. The total mass of undissolved crystals remains constant. C. When the rate of solvation equals the rate of crystallization, a state of dynamic equilibrium exists. D. If more solute were added to the con ...
Cl3CCN/PPh3 and CBr4/PPh3: two efficient reagent systems for the
... bromides. 2-Hydroxypyridine was used as a model substrate and the yield of the product, 2-bromopyridine was quantified by HPLC (see Table 3). On refluxing in toluene for 4 h, the reaction of 2-hydroxypyridine with Br3CCO2Et and NBS provided the desired bromides in 3% and 25% yields, respectively (entr ...
... bromides. 2-Hydroxypyridine was used as a model substrate and the yield of the product, 2-bromopyridine was quantified by HPLC (see Table 3). On refluxing in toluene for 4 h, the reaction of 2-hydroxypyridine with Br3CCO2Et and NBS provided the desired bromides in 3% and 25% yields, respectively (entr ...
Notes 10
... Reactions are conveniently classified as substitutions, additions, eliminations and rearrangements. These terms describe the overall process, simply comparing the structure of starting materials and products. They do not indicate anything about the pathway (“mechanism”) by which the reaction proceed ...
... Reactions are conveniently classified as substitutions, additions, eliminations and rearrangements. These terms describe the overall process, simply comparing the structure of starting materials and products. They do not indicate anything about the pathway (“mechanism”) by which the reaction proceed ...
04 Reactions in Aqueous Solution
... electrolytes (strong acids, strong bases, and soluble ionic salts) are dissociated into their ions. • This more accurately reflects the species that are found in the reaction mixture. Ag+(aq) + NO3−(aq) + K+(aq) + Cl−(aq) AgCl(s) + K+(aq) + NO3−(aq) ...
... electrolytes (strong acids, strong bases, and soluble ionic salts) are dissociated into their ions. • This more accurately reflects the species that are found in the reaction mixture. Ag+(aq) + NO3−(aq) + K+(aq) + Cl−(aq) AgCl(s) + K+(aq) + NO3−(aq) ...
AP Chemistry Lab Manual
... working document, not a perfect, error-free, polished product. Errors should be corrected by drawing one line through the mistake, and then proceeding with the new data. 6. Do not use the first person or include personal comments. Prelab Instructions: 1. On most every lab you will have prelab instru ...
... working document, not a perfect, error-free, polished product. Errors should be corrected by drawing one line through the mistake, and then proceeding with the new data. 6. Do not use the first person or include personal comments. Prelab Instructions: 1. On most every lab you will have prelab instru ...
File
... working document, not a perfect, error-free, polished product. Errors should be corrected by drawing one line through the mistake, and then proceeding with the new data. 6. Do not use the first person or include personal comments. Prelab Instructions: 1. On most every lab you will have prelab instru ...
... working document, not a perfect, error-free, polished product. Errors should be corrected by drawing one line through the mistake, and then proceeding with the new data. 6. Do not use the first person or include personal comments. Prelab Instructions: 1. On most every lab you will have prelab instru ...
PAGE PROOFS
... Neutralisation is the process of an acid reacting with a base. The acid and base properties can be cancelled out if the exact amounts of both are used. A neutralisation reaction produces a salt and water as its products. All neutralisation reactions can be represented by the following ionic equation ...
... Neutralisation is the process of an acid reacting with a base. The acid and base properties can be cancelled out if the exact amounts of both are used. A neutralisation reaction produces a salt and water as its products. All neutralisation reactions can be represented by the following ionic equation ...
Mole-Volume Conversion Assignment
... 6. Add 10 – 15 mL of distilled water to each 100 mL beaker. Gently swirl the contents of the beakers to dissolve the solids. All solid should be dissolved before you proceed (this will take 1 – 2 ...
... 6. Add 10 – 15 mL of distilled water to each 100 mL beaker. Gently swirl the contents of the beakers to dissolve the solids. All solid should be dissolved before you proceed (this will take 1 – 2 ...
Chromatographic and Mass Spectral Studies on Isobaric and
... fragment of m/z 135. A complete set of the ring and side-chain regioisomers of the methoxymethylphenethylamines will likely produce several compounds similar in retention properties to compound 3, MDMA. When more polar columns (DB-35MS and HP50+) were applied for the separation, 3,4-MDMA coeluted wi ...
... fragment of m/z 135. A complete set of the ring and side-chain regioisomers of the methoxymethylphenethylamines will likely produce several compounds similar in retention properties to compound 3, MDMA. When more polar columns (DB-35MS and HP50+) were applied for the separation, 3,4-MDMA coeluted wi ...
CH_12_3_Reactions_Alcohols_Thiols
... • there is an increase in the number of C–O bonds • there is a loss of H In a reduction, • there is a decrease in the number of C–O bonds • there is a gain of H ...
... • there is an increase in the number of C–O bonds • there is a loss of H In a reduction, • there is a decrease in the number of C–O bonds • there is a gain of H ...
Alkenes notes
... Eg CH2=CH2 + Br2 CH2BrCH2Br The Br-Br molecule is non-polar but in the presence of alkenes the electrons move to one side of the molecule and it acquires a temporary dipole (in other words a dipole is induced by the alkene). The alkene: C ...
... Eg CH2=CH2 + Br2 CH2BrCH2Br The Br-Br molecule is non-polar but in the presence of alkenes the electrons move to one side of the molecule and it acquires a temporary dipole (in other words a dipole is induced by the alkene). The alkene: C ...
Organic Chemistry/Fourth Edition: e-Text
... In the Strecker synthesis an aldehyde is treated with ammonia and a source of cyanide ion. The resulting amino nitrile is hydrolyzed to an amino acid. O NH3 ...
... In the Strecker synthesis an aldehyde is treated with ammonia and a source of cyanide ion. The resulting amino nitrile is hydrolyzed to an amino acid. O NH3 ...
S. Y. B. Sc. Chemistry
... Chapter 3 : Chemistry of Natural and Unnatural carboxylic acids and their derivatives i) Natural and unnatural carboxylic acid ii) Write functional group of acid and their derivatives iii) Explain physical and chemical properties of carboxylic acid iv) To write and complete various reactions of carb ...
... Chapter 3 : Chemistry of Natural and Unnatural carboxylic acids and their derivatives i) Natural and unnatural carboxylic acid ii) Write functional group of acid and their derivatives iii) Explain physical and chemical properties of carboxylic acid iv) To write and complete various reactions of carb ...
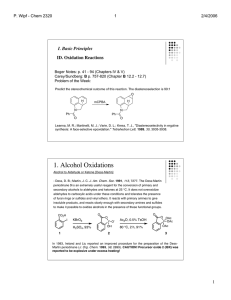
1. Alcohol Oxidations
... For a catalytic protocol, see: Bolm, C.; Magnus, A. S.; Hildebrand, J. P., "Catalytic synthesis of aldehydes and ketones under mild conditions using TEMPO/oxone." Org. Lett. 2000, 2, 1173-1175. TEMPO/NCS allows for the selective oxidation of primary over secondary alcohols: Einhorn, J.; Einhorn, C.; ...
... For a catalytic protocol, see: Bolm, C.; Magnus, A. S.; Hildebrand, J. P., "Catalytic synthesis of aldehydes and ketones under mild conditions using TEMPO/oxone." Org. Lett. 2000, 2, 1173-1175. TEMPO/NCS allows for the selective oxidation of primary over secondary alcohols: Einhorn, J.; Einhorn, C.; ...
getting started 3.1 hydrocarbons
... and alkynes makes them more reactive than alkanes, which have only single bonds. 4. saturated hydrocarbon: ...
... and alkynes makes them more reactive than alkanes, which have only single bonds. 4. saturated hydrocarbon: ...
stoker-C15
... (2) An alkoxy group and a hydroxy group attached to the same carbon atom are present in both hemiacetals and acetals. (3) Cyclic aldehyde structures are possible but cyclic ketone structures are not possible. A) All three statements are true. B) Two of the three statements are true. C) Only one of t ...
... (2) An alkoxy group and a hydroxy group attached to the same carbon atom are present in both hemiacetals and acetals. (3) Cyclic aldehyde structures are possible but cyclic ketone structures are not possible. A) All three statements are true. B) Two of the three statements are true. C) Only one of t ...
LECTURE 7 REDUCTIVE ELIMINATIONSa
... product was determined by carbonylation to give the metal acyl followed by methanolysis to give the ester. • Both of these reactions are known to leave the configuration at carbon unchanged, and the configuration of the ester can be determined unambiguously from the measured optical rotation of the ...
... product was determined by carbonylation to give the metal acyl followed by methanolysis to give the ester. • Both of these reactions are known to leave the configuration at carbon unchanged, and the configuration of the ester can be determined unambiguously from the measured optical rotation of the ...
Net ionic equation
... The forces holding an ionic compound together are the strong electrical attraction that exists between cations and anions. It is therefore somewhat surprising that ionic compounds will dissolve in water. The reason some ionic compounds will dissolve in water is because the water molecules have a par ...
... The forces holding an ionic compound together are the strong electrical attraction that exists between cations and anions. It is therefore somewhat surprising that ionic compounds will dissolve in water. The reason some ionic compounds will dissolve in water is because the water molecules have a par ...
CHM 235 Course Outline and Homework in McMurry (6th ed.)
... Reactions of Grignards and Gilman reagents (rxns 2c and 2d) Oxidation/reduction reactions in organic chemistry Chapter 11: 11.25-11.31, 11.34-11.38, 11.41, 11.45-11.47, 11.50, 11.54, 11.55, 11.5911.60, 11.68 The SN2 substitution reaction (stereochemistry, kinetics, the substrate, the attacking ...
... Reactions of Grignards and Gilman reagents (rxns 2c and 2d) Oxidation/reduction reactions in organic chemistry Chapter 11: 11.25-11.31, 11.34-11.38, 11.41, 11.45-11.47, 11.50, 11.54, 11.55, 11.5911.60, 11.68 The SN2 substitution reaction (stereochemistry, kinetics, the substrate, the attacking ...
Question Bank Topic 5
... (CDC guide: Simple chemical cells: (a) consisting of two metal electrodes and and electrolyte, (b) consisting of metal-metal ion half-cells and salt bridge / porous device; Changes occurring at the electrodes and electron flow in the external circuit; Ionic half-equations and overall cell equations) ...
... (CDC guide: Simple chemical cells: (a) consisting of two metal electrodes and and electrolyte, (b) consisting of metal-metal ion half-cells and salt bridge / porous device; Changes occurring at the electrodes and electron flow in the external circuit; Ionic half-equations and overall cell equations) ...
M.Sc - I Syllabus w.e.f. June 2015
... determination from Freezing point depression and vapour-pressure measurements. Third law entropies- Calculations of standard entropies from Cp data, Exceptions to ...
... determination from Freezing point depression and vapour-pressure measurements. Third law entropies- Calculations of standard entropies from Cp data, Exceptions to ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.