Derivatives of Carboxylic Acids
... Amides • Preparation of Esters : condensation reaction between alcohol and acid O ...
... Amides • Preparation of Esters : condensation reaction between alcohol and acid O ...
Document
... The Fischer esterification is conducted at reflux. The purpose of reflux is to heat a reaction mixture at its boiling temperature to form products, without losing any of the compounds in the reaction flask. In practice, a condenser is set vertically into the top of the reaction flask. Any compound t ...
... The Fischer esterification is conducted at reflux. The purpose of reflux is to heat a reaction mixture at its boiling temperature to form products, without losing any of the compounds in the reaction flask. In practice, a condenser is set vertically into the top of the reaction flask. Any compound t ...
- KCN K+ R KOH + H2O
... Remember, RBr ⇒ ROH; and we have seen that RCHO or R2CO ⇒ R”CH2OH or R”2CHOH (oxidation of aldehydes and ketones) Which starting materials would you use to prepare PhCH=C(CH3)2? PhCHO and (CH3)2CHBr versus PhCH2Br and (CH3)2CO? How would you prepare PhCH2Br from PhCOOMe? How would you prepare PhCHO ...
... Remember, RBr ⇒ ROH; and we have seen that RCHO or R2CO ⇒ R”CH2OH or R”2CHOH (oxidation of aldehydes and ketones) Which starting materials would you use to prepare PhCH=C(CH3)2? PhCHO and (CH3)2CHBr versus PhCH2Br and (CH3)2CO? How would you prepare PhCH2Br from PhCOOMe? How would you prepare PhCHO ...
study guide and review for first semester final
... from percentage composition to determine the compound’s empirical formula. Ex. A compound with 0.90 g Ca and 1.6 g Cl has what empirical formula? (CaCl2) Ex. A white powder used in paints, enamels and ceramics has the following percentage composition: Ba 69.6 %; C 6.09%; O 24.3 %. What is its empiri ...
... from percentage composition to determine the compound’s empirical formula. Ex. A compound with 0.90 g Ca and 1.6 g Cl has what empirical formula? (CaCl2) Ex. A white powder used in paints, enamels and ceramics has the following percentage composition: Ba 69.6 %; C 6.09%; O 24.3 %. What is its empiri ...
info
... i. NaBH4 will reduce an aldehyde, ketone, or acid chloride to the corresponding alcohol. It will not reduce an acid or an ester. ii. LiAlH4 will reduce an aldehyde, ketone, acid, or ester to the corresponding alcohol. iii. LiAlH(OtBu)3 will reduce an acid chloride to an aldehyde. iv. DIBAL will ...
... i. NaBH4 will reduce an aldehyde, ketone, or acid chloride to the corresponding alcohol. It will not reduce an acid or an ester. ii. LiAlH4 will reduce an aldehyde, ketone, acid, or ester to the corresponding alcohol. iii. LiAlH(OtBu)3 will reduce an acid chloride to an aldehyde. iv. DIBAL will ...
CHAPTER 12 Solid-Phase Synthesis of Peptides Containing the
... by Hamada and Shiori (3) utilizes a combination of sulfur trioxide-pyridine complex and DMSO in the presence of TEA (also known as the Parikh-Doering oxidation) to accomplish this oxidation (Fig. 3). 1. Dissolve the protected a-amino alcohol (10 mmol) in 30 mL of anhydrous DMSO containing TEA (3.035 ...
... by Hamada and Shiori (3) utilizes a combination of sulfur trioxide-pyridine complex and DMSO in the presence of TEA (also known as the Parikh-Doering oxidation) to accomplish this oxidation (Fig. 3). 1. Dissolve the protected a-amino alcohol (10 mmol) in 30 mL of anhydrous DMSO containing TEA (3.035 ...
Pre-AP Chemistry
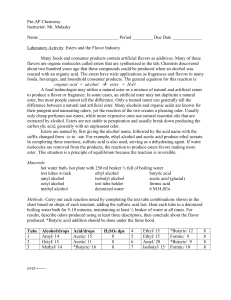
... Many foods and consumer products contain artificial flavors as additives. Many of these flavors are organic molecules called esters that are synthesized in the lab. Chemists discovered about two hundred years ago that these compounds could be produced when an alcohol was reacted with an organic acid ...
... Many foods and consumer products contain artificial flavors as additives. Many of these flavors are organic molecules called esters that are synthesized in the lab. Chemists discovered about two hundred years ago that these compounds could be produced when an alcohol was reacted with an organic acid ...
CHM412 June 2013 paper
... the strong hydrogen bonds), then molecuels with dipoles which have lower bpt’s followed by (non-polar) molecules with LDF’s only. Within the H-bonding species, Carboxylic acids form dimers (as part d) mentions), that is, two sets of Hbonding to one other molecule, so their H-bonds are stronger than ...
... the strong hydrogen bonds), then molecuels with dipoles which have lower bpt’s followed by (non-polar) molecules with LDF’s only. Within the H-bonding species, Carboxylic acids form dimers (as part d) mentions), that is, two sets of Hbonding to one other molecule, so their H-bonds are stronger than ...
Rapid, Controlled Assembly of Polyenes for Studying Pericyclic
... Rapid, Controlled Assembly of Polyenes for Studying Pericyclic Reaction Cascades David A. Vosburg, Department of Chemistry, Harvey Mudd College Pericyclic reactions are among the most powerful transformations in organic chemistry, and they are even more impressive when they occur in tandem. Outstand ...
... Rapid, Controlled Assembly of Polyenes for Studying Pericyclic Reaction Cascades David A. Vosburg, Department of Chemistry, Harvey Mudd College Pericyclic reactions are among the most powerful transformations in organic chemistry, and they are even more impressive when they occur in tandem. Outstand ...
Functional Groups - World of Teaching
... thousand powerpoints submitted by teachers. This is a completely free site and requires no registration. Please visit and I hope it will help in your teaching. ...
... thousand powerpoints submitted by teachers. This is a completely free site and requires no registration. Please visit and I hope it will help in your teaching. ...
Aldehydes and Ketones Both contain the functional group C O
... Of the two reducing agents, sodium borohydride is the milder reagent and is the one of preference for aldehydes and ketones since it is specific for these two functional groups. Lithium aluminum hydride will reduce many types of compounds very quickly. Reduction to hydrocarbons (we saw this used to ...
... Of the two reducing agents, sodium borohydride is the milder reagent and is the one of preference for aldehydes and ketones since it is specific for these two functional groups. Lithium aluminum hydride will reduce many types of compounds very quickly. Reduction to hydrocarbons (we saw this used to ...
Reactions of Alkenes Organic Chemistry
... Note: These examples were adapted and revised from General, Organic, & Biological Chemistry textbook (with author: Janice Gorzynski Smith) ...
... Note: These examples were adapted and revised from General, Organic, & Biological Chemistry textbook (with author: Janice Gorzynski Smith) ...
... hydrobenzofurans 2 from homobenzylic alcohols 1 via an intramolecular palladium-catalyzed C–H activation/C–O cyclization sequence. Both EDGs and EWGs are tolerated at various positions around the ring. Yields are excellent (>75%) in almost all cases, except for R3 = CO2Et (50%) and R3 = H (42%). The ...
Revision
... A C7H13Br compound reacts with KOH in ethanol to form 3methylcyclohexene as the major product. What is a likely structure for the starting alkyl bromide? ...
... A C7H13Br compound reacts with KOH in ethanol to form 3methylcyclohexene as the major product. What is a likely structure for the starting alkyl bromide? ...
Exam - Chemistry With BT
... requires more than one step. Show all the steps of the synthesis in the right sequence. Give the reagents used and the reaction conditions utilized (including acid base catalysis). Show the structures of all intermediate products. ...
... requires more than one step. Show all the steps of the synthesis in the right sequence. Give the reagents used and the reaction conditions utilized (including acid base catalysis). Show the structures of all intermediate products. ...
synthesizing esters in the laboratory
... artificial strawberry that smells completely "real" has yet to be synthesized since there are dozens of other molecules mixed in but they have yet to be identified. Vanilla is an orchid endemic to South America. In comparing the infrared spectrographs of vanilla extract and artificial vanilla, a str ...
... artificial strawberry that smells completely "real" has yet to be synthesized since there are dozens of other molecules mixed in but they have yet to be identified. Vanilla is an orchid endemic to South America. In comparing the infrared spectrographs of vanilla extract and artificial vanilla, a str ...
Q1. Give I.U.P.A..C Name of the following Organic Compound. 1 CH
... Q5. What is the formula of a crystalline Compound in which atoms A are present at all the eight corners and atom B at the centre of all side faces? ...
... Q5. What is the formula of a crystalline Compound in which atoms A are present at all the eight corners and atom B at the centre of all side faces? ...
Synthesis/Decomposition Reactions
... In these reactions, two different molecules or atoms combine to form a single substance. ...
... In these reactions, two different molecules or atoms combine to form a single substance. ...
Document
... List three characteristics of an homologous series, and explain the term functional group. ...
... List three characteristics of an homologous series, and explain the term functional group. ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.