Coordination Chemistry
... screw into the plane while the Λ isomer appears to screw out of the plane (Figure 4.10d). Thus [Co(en)3]3+ is chiral, and can be resolved into its ∆ and Λ isomers through fractional crystallization with a chiral counter ion such as tartarate. A tetrahedral complex with four different unidentate liga ...
... screw into the plane while the Λ isomer appears to screw out of the plane (Figure 4.10d). Thus [Co(en)3]3+ is chiral, and can be resolved into its ∆ and Λ isomers through fractional crystallization with a chiral counter ion such as tartarate. A tetrahedral complex with four different unidentate liga ...
FTIR Spectoscopy of Ca++ DOPED Alq3 OLED Phospor
... quantum efficiency. Alq3 is used as electron transporting layer, as emission layer where green light emission is generated by electron hole recombination in Alq3. It is also served as host material for various dyes to tune emission color from red to green [3]. Although Alq3 has low fluorescence effi ...
... quantum efficiency. Alq3 is used as electron transporting layer, as emission layer where green light emission is generated by electron hole recombination in Alq3. It is also served as host material for various dyes to tune emission color from red to green [3]. Although Alq3 has low fluorescence effi ...
Effect of nucleophile on reaction
... • The stronger the nucleophile the faster / more efficient the SN2 reaction • Nucleophilic strength (nucleophilicity) relates to how easily a compound can donate an electron pair • The more electronegative an atom the less nucleophilic as the electrons are held closer Anion more nucleophilic than it ...
... • The stronger the nucleophile the faster / more efficient the SN2 reaction • Nucleophilic strength (nucleophilicity) relates to how easily a compound can donate an electron pair • The more electronegative an atom the less nucleophilic as the electrons are held closer Anion more nucleophilic than it ...
Ch 25 Hydrocarbon Compounds
... • b) H2SO4 is the dehydrating agent. It removes H from one alcohol molecule and OH from the other to form H2O. ...
... • b) H2SO4 is the dehydrating agent. It removes H from one alcohol molecule and OH from the other to form H2O. ...
p-HYDROXYBENZOATE COMPLEXES OF Ni(II), Cu(II) AND Zn(II)
... The absorption bands in the range of 3472–3431 cm–1 in complexes correspond to the asymmetric and symmetric stretching vibration of water molecules. The wave number range for the N–H stretches of primary amides is 3370–3170 cm–1. We observed two bands in the range of 3350–3245 cm–1 in the na complex ...
... The absorption bands in the range of 3472–3431 cm–1 in complexes correspond to the asymmetric and symmetric stretching vibration of water molecules. The wave number range for the N–H stretches of primary amides is 3370–3170 cm–1. We observed two bands in the range of 3350–3245 cm–1 in the na complex ...
carboxylic acid
... Recall and explain the physical properties of carboxylic acids Recall the structures of carboxylic acids, esters and acyl chlorides Recall the acidic properties of carboxylic acids Recall and explain the esterification of carboxylic acids Write balanced equations representing any reactions in the se ...
... Recall and explain the physical properties of carboxylic acids Recall the structures of carboxylic acids, esters and acyl chlorides Recall the acidic properties of carboxylic acids Recall and explain the esterification of carboxylic acids Write balanced equations representing any reactions in the se ...
Nuclear Debate - Noadswood Science
... However the product is impure as concentrations higher than 15% denature the enzymes killing the yeast. To purify it distillation is used, which uses energy. ...
... However the product is impure as concentrations higher than 15% denature the enzymes killing the yeast. To purify it distillation is used, which uses energy. ...
Experiment 1. Formation of silver thiosulphate complex
... coordinate covalent bonds. The formula for this complex ion is Co(NH3)63+. The central metal ion with its attached ligands is called the coordination sphere. In the neutral compound with the empirical formula CoCl3 ∙ 6NH3, each Co(NH3)63+ ion is associated with three chloride ions. Thus, the formula ...
... coordinate covalent bonds. The formula for this complex ion is Co(NH3)63+. The central metal ion with its attached ligands is called the coordination sphere. In the neutral compound with the empirical formula CoCl3 ∙ 6NH3, each Co(NH3)63+ ion is associated with three chloride ions. Thus, the formula ...
kinetics, catalysis, and reaction engineering
... was used to determine the main effects and two-factor interactions for the factors of C3H6 concentration, NO concentration, temperature, and GHSV on HCN conversion. A table of contrast was used to estimate the significance of these factors. The specific levels (lowest and highest values), HCN conver ...
... was used to determine the main effects and two-factor interactions for the factors of C3H6 concentration, NO concentration, temperature, and GHSV on HCN conversion. A table of contrast was used to estimate the significance of these factors. The specific levels (lowest and highest values), HCN conver ...
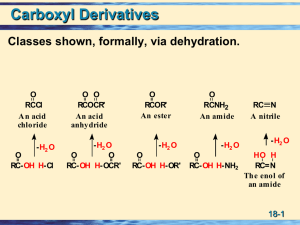
Chapter 18 Carboxylic Acid Derivatives
... readily with water to give two molecules of carboxylic acid. – Higher-molecular-weight anhydrides also react with water, but less readily. O O CH3 COCCH3 + H2 O ...
... readily with water to give two molecules of carboxylic acid. – Higher-molecular-weight anhydrides also react with water, but less readily. O O CH3 COCCH3 + H2 O ...
organic problems - St. Olaf College
... 25 Which of the following molecular formulas is reasonable for a stable compound? A) C8H14O2Cl B) C6H14Br2 C) C7H10NF D) C30H54N2Cl 26 What formal charges are present in the molecule C6H5C≡N-O? ( all heavy atoms have a valence shell octet, and C6H5- is a phenyl group) A) N is -1 and C is +1 B) N is ...
... 25 Which of the following molecular formulas is reasonable for a stable compound? A) C8H14O2Cl B) C6H14Br2 C) C7H10NF D) C30H54N2Cl 26 What formal charges are present in the molecule C6H5C≡N-O? ( all heavy atoms have a valence shell octet, and C6H5- is a phenyl group) A) N is -1 and C is +1 B) N is ...
Organic synthesis and methodology related to the malaria drug artemisinin
... Figure 64: Initial Substrate Screening with MeCN ..................................... 121 Figure 65: Initial Substrate Screening with CH2Cl2 .................................... 122 Figure 66: Use of Electron-Rich Ethoxyacetylene ..................................... 123 Figure 67: Qualitative Scre ...
... Figure 64: Initial Substrate Screening with MeCN ..................................... 121 Figure 65: Initial Substrate Screening with CH2Cl2 .................................... 122 Figure 66: Use of Electron-Rich Ethoxyacetylene ..................................... 123 Figure 67: Qualitative Scre ...
pdf
... Weinreb, S. W. et al. Tetrahedron Le=. 1977, 18, 4171. Dias, L. C. et al. Org. Le=. 2003, 5, 265. Evans, D. A. et al. J. Am. Chem. Soc. 2002, 124, 392. ...
... Weinreb, S. W. et al. Tetrahedron Le=. 1977, 18, 4171. Dias, L. C. et al. Org. Le=. 2003, 5, 265. Evans, D. A. et al. J. Am. Chem. Soc. 2002, 124, 392. ...
Metal-Catalyzed Epoxidations of Alkenes with Hydrogen
... 1-type zeolites are inherently acidic, they are sometimes modified prior to use to prevent inactivation of the catalyst or epoxide-decomposition. Even with such modifications, zeolites are generally limited to production of small, fairly stable epoxides. Hydrotalcite systems are more widely applicab ...
... 1-type zeolites are inherently acidic, they are sometimes modified prior to use to prevent inactivation of the catalyst or epoxide-decomposition. Even with such modifications, zeolites are generally limited to production of small, fairly stable epoxides. Hydrotalcite systems are more widely applicab ...
Problem 1: A brief history of life in the universe
... boiling point of water due to continued bombardment by asteroids. When the Earth cooled, it rained and a primitive ocean was formed. As metals and their salts dissolved the ocean became alkaline and a large amount of carbon dioxide from the air dissolved in the ocean. The CO2 part of most carbonate ...
... boiling point of water due to continued bombardment by asteroids. When the Earth cooled, it rained and a primitive ocean was formed. As metals and their salts dissolved the ocean became alkaline and a large amount of carbon dioxide from the air dissolved in the ocean. The CO2 part of most carbonate ...
Problem 1: “A brief history” of life in the universe
... boiling point of water due to continued bombardment by asteroids. When the Earth cooled, it rained and a primitive ocean was formed. As metals and their salts dissolved the ocean became alkaline and a large amount of carbon dioxide from the air dissolved in the ocean. The CO2 part of most carbonate ...
... boiling point of water due to continued bombardment by asteroids. When the Earth cooled, it rained and a primitive ocean was formed. As metals and their salts dissolved the ocean became alkaline and a large amount of carbon dioxide from the air dissolved in the ocean. The CO2 part of most carbonate ...
Transport of metal ions across polymer inclusion membrane with 1
... bonding is invariable in the Cu(II) complexes with all the 1-alkylimidazoles. The initial fluxes of Zn(II) ions increase more rapidly than Co(II) and Ni(II) ions with increase of pKa carriers. The values of the diffusion coefficient of metal complex in the membrane as well as the constant rate of re ...
... bonding is invariable in the Cu(II) complexes with all the 1-alkylimidazoles. The initial fluxes of Zn(II) ions increase more rapidly than Co(II) and Ni(II) ions with increase of pKa carriers. The values of the diffusion coefficient of metal complex in the membrane as well as the constant rate of re ...
ether - HCC Southeast Commons
... are prepared by the sulfuric acid-catalyzed dehydration procedure? What product(s) would you expect if ethanol and 1-propanol were allowed to react together? In what ratio would the products be formed if the two alcohols were of equal reactivity? ...
... are prepared by the sulfuric acid-catalyzed dehydration procedure? What product(s) would you expect if ethanol and 1-propanol were allowed to react together? In what ratio would the products be formed if the two alcohols were of equal reactivity? ...
Part II
... Free radicals – have unpaired electron(s). Atmospheric lifetimes seconds, minutes. e.g., •O-H radical, missing one bond (H), wants to steal one from somewhere. Similar story for •CH3 radical, missing one bond. Or the HO2 radical, H-O-O• These free radicals are usually generated by sunlight (photoche ...
... Free radicals – have unpaired electron(s). Atmospheric lifetimes seconds, minutes. e.g., •O-H radical, missing one bond (H), wants to steal one from somewhere. Similar story for •CH3 radical, missing one bond. Or the HO2 radical, H-O-O• These free radicals are usually generated by sunlight (photoche ...
QUESTION BANK CHEMISTRY-XII THE SOLID STATE CHAPTER
... 41. Explain the term electrolysis .Write the reactions at cathode and anode when following substances are electrolysed :‐ (i) Molten NaCl (ii) Aqueous solution of NaCl 42. What is meant by electrochemical series? How does it help in :‐ (i) comparing the relative oxidizing or reduci ...
... 41. Explain the term electrolysis .Write the reactions at cathode and anode when following substances are electrolysed :‐ (i) Molten NaCl (ii) Aqueous solution of NaCl 42. What is meant by electrochemical series? How does it help in :‐ (i) comparing the relative oxidizing or reduci ...
Studying of transition metal complexes containing
... temperature and incubated at 37°C for 24 hr . The antibacterial activity was evaluated by measuring the diameter of the inhibition zone (I.Z) around the hole in millimeter m.m(19) . Results and Discussion The physical properties of ligands and the complexes were shown in table (1) as : Table (1): ph ...
... temperature and incubated at 37°C for 24 hr . The antibacterial activity was evaluated by measuring the diameter of the inhibition zone (I.Z) around the hole in millimeter m.m(19) . Results and Discussion The physical properties of ligands and the complexes were shown in table (1) as : Table (1): ph ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.