Metal–organic complexation in the marine environment | SpringerLink
... Fe(III), the Kcond Fe(III)L of a Fe–natural ligand complex and total natural ligand concentration [CL] can be calculated from the intercept and slope of a [Felabile]/[FeL] vs. [Felabile] plot. [Felabile] is that metal that can bind with the competitive ligand and is obtained from the CSV Fe peak cur ...
... Fe(III), the Kcond Fe(III)L of a Fe–natural ligand complex and total natural ligand concentration [CL] can be calculated from the intercept and slope of a [Felabile]/[FeL] vs. [Felabile] plot. [Felabile] is that metal that can bind with the competitive ligand and is obtained from the CSV Fe peak cur ...
Chapter 22 and 23 Study Guide
... You should review homework, quizzes and other assignments for complete test preparation. These questions are only examples. Different chemicals will be on the test. ...
... You should review homework, quizzes and other assignments for complete test preparation. These questions are only examples. Different chemicals will be on the test. ...
Design and Construction of Coordination Polymers Brochure
... 4.2 Complexes Built Up by POMs with 1,2,4–Triazolate and Its Derivatives. 4.3 Complexes Built Up by Molybdenum Oxide Chains with Pyridine Derivatives. 4.4 Conclusions. References. 5 Silver(I) Coordination Polymers (Cheng–Yong Su, Chun–Long Chen, Jian–Yong Zhang, and Bei–Sheng Kang). ...
... 4.2 Complexes Built Up by POMs with 1,2,4–Triazolate and Its Derivatives. 4.3 Complexes Built Up by Molybdenum Oxide Chains with Pyridine Derivatives. 4.4 Conclusions. References. 5 Silver(I) Coordination Polymers (Cheng–Yong Su, Chun–Long Chen, Jian–Yong Zhang, and Bei–Sheng Kang). ...
Boston University Dresden Science Program Instructor: Meeting Times
... Lecture Textbooks and Other Course Material.: Organic Chemistry, Boston University, Custom 7e by William H. Brown et. all., Cengage Learning 2013, Solutions Manual, 6th Edition, Cencage Learning 2012, and Pushing Electrons, by Daniel Weeks (Saunders) - this book is recommended but not required. Mole ...
... Lecture Textbooks and Other Course Material.: Organic Chemistry, Boston University, Custom 7e by William H. Brown et. all., Cengage Learning 2013, Solutions Manual, 6th Edition, Cencage Learning 2012, and Pushing Electrons, by Daniel Weeks (Saunders) - this book is recommended but not required. Mole ...
Scope and Limitations - Organic Reactions Wiki
... amounts of transition metal due to the inability of the resulting osmate(VI) ester to undergo either direct hydrolysis or in situ oxidation to a more easily hydrolyzed Os(VIII) species. By switching to monodentate amines such as quinuclidine, introduced as its Noxide (QNO), the reactivity and hydrog ...
... amounts of transition metal due to the inability of the resulting osmate(VI) ester to undergo either direct hydrolysis or in situ oxidation to a more easily hydrolyzed Os(VIII) species. By switching to monodentate amines such as quinuclidine, introduced as its Noxide (QNO), the reactivity and hydrog ...
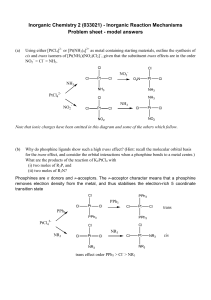
Inorganic Chemistry 2 (033021) - Inorganic Reaction
... This question comes from Shriver and Atkins, who give the answer: Ir(III) < Rh(III) < Co(III) < Ni(II) < Mn(II). Mn(II) is d5 and high spin, so no CFSE. Ni(II) is d8 and there is a large loss of CFSE on going to any 5 coordinate geometry, so there is a significant CFAE. The other 3 are all d6 low sp ...
... This question comes from Shriver and Atkins, who give the answer: Ir(III) < Rh(III) < Co(III) < Ni(II) < Mn(II). Mn(II) is d5 and high spin, so no CFSE. Ni(II) is d8 and there is a large loss of CFSE on going to any 5 coordinate geometry, so there is a significant CFAE. The other 3 are all d6 low sp ...
3 Properties Alcohols GOB Structures
... • the oxygen has a partially negative charge. • the hydrogen has a partially positive charge. • hydrogen bonds form between the oxygen of one alcohol and hydrogen in the — OH group of another alcohol. ...
... • the oxygen has a partially negative charge. • the hydrogen has a partially positive charge. • hydrogen bonds form between the oxygen of one alcohol and hydrogen in the — OH group of another alcohol. ...
Pentadienyl Complexes of Alkali Metals
... pentadienylsodium (C5H7Na) complexes.19 They determined that the U-shaped pentadienyl structure is the most stable conformation for both complexes. The preference in these metal pentadienyl compounds, C5H7E, for one conformer over another was explained by the extents of electron delocalization in th ...
... pentadienylsodium (C5H7Na) complexes.19 They determined that the U-shaped pentadienyl structure is the most stable conformation for both complexes. The preference in these metal pentadienyl compounds, C5H7E, for one conformer over another was explained by the extents of electron delocalization in th ...
Word - icho39.chem.msu.ru
... Let nH 2 = nH2 H2 , where H 2 is the number of moles of hydrogen added to the system. Since H 2 is small, H2 ...
... Let nH 2 = nH2 H2 , where H 2 is the number of moles of hydrogen added to the system. Since H 2 is small, H2 ...
Slide 1
... The more electronegative an element is the more it will pull the electrons of the other element towards it. This apparent charge is called an oxidation number, which is the charge it would have if it gained or lost electrons. ...
... The more electronegative an element is the more it will pull the electrons of the other element towards it. This apparent charge is called an oxidation number, which is the charge it would have if it gained or lost electrons. ...
Academic Year 2009/2010 Semester I KTT 212/3 Inorganic
... An overview of the background and basic aspects related to the coordination compounds or complexes which include the definition, nomenclature based on IUPAC system, coordination number, oxidation state for central metal atom, types of ligands and complexes. Establishment of different structures owin ...
... An overview of the background and basic aspects related to the coordination compounds or complexes which include the definition, nomenclature based on IUPAC system, coordination number, oxidation state for central metal atom, types of ligands and complexes. Establishment of different structures owin ...
Linear hexadentate ligands as iron chelators
... strategy was used successfully to prepare 5LIHOPO2TAM (7) and 6LIHOPO2TAM (8). When the benzyl-protected TAM starting material 11 was used, it was also possible to prepare 3LIHOPO2TAM (5) using this method. When preparing 5LIOHOPO2TAM (9), a modified strategy was required, as it was necessary first ...
... strategy was used successfully to prepare 5LIHOPO2TAM (7) and 6LIHOPO2TAM (8). When the benzyl-protected TAM starting material 11 was used, it was also possible to prepare 3LIHOPO2TAM (5) using this method. When preparing 5LIOHOPO2TAM (9), a modified strategy was required, as it was necessary first ...
Document
... Main constituent of vinegar and is obtained by fermentation of molasses in presence of air. Industrially, it is obtained in pure form by oxidation of ethanal with air in the presence of cobalt acetate catalyst or by carbonylation of methanol in the presence of rhodium catalyst. ...
... Main constituent of vinegar and is obtained by fermentation of molasses in presence of air. Industrially, it is obtained in pure form by oxidation of ethanal with air in the presence of cobalt acetate catalyst or by carbonylation of methanol in the presence of rhodium catalyst. ...
22.4: Acidity of Phenols.
... to an sp3-hybridized carbon. Phenols contain an OH group bonded to an sp2-hybridized carbon of a benzene ring 22.1: Nomenclature (please read) 22.2: Structure and Bonding (please read) 22.3: Physical Properties (please read). Like other alcohols the OH group of phenols cab participate in hydrogen bo ...
... to an sp3-hybridized carbon. Phenols contain an OH group bonded to an sp2-hybridized carbon of a benzene ring 22.1: Nomenclature (please read) 22.2: Structure and Bonding (please read) 22.3: Physical Properties (please read). Like other alcohols the OH group of phenols cab participate in hydrogen bo ...
A Mild and Convenient Conversion of Ketones to the Corresponding
... One of the most versatile of these procedures involves the use of catecholborane as the reducing agent.2 The catecholboranetosylhydrazone procedure offers a number of advantages over methods utilizing sodium borohydride3p5 and sodium cyanoborohydride! These advantages include (a) efficient use of hy ...
... One of the most versatile of these procedures involves the use of catecholborane as the reducing agent.2 The catecholboranetosylhydrazone procedure offers a number of advantages over methods utilizing sodium borohydride3p5 and sodium cyanoborohydride! These advantages include (a) efficient use of hy ...
52142_present
... Hydrazones played a central role in the development of coordination chemistry. Hydrazones obtained by the condensation of o-hydroxy or methoxy aldehydes and ketones with hydrazides are considered potential polynucleating ligands because they posses amide, azomethine and phenol or methoxy functions t ...
... Hydrazones played a central role in the development of coordination chemistry. Hydrazones obtained by the condensation of o-hydroxy or methoxy aldehydes and ketones with hydrazides are considered potential polynucleating ligands because they posses amide, azomethine and phenol or methoxy functions t ...
Characterization and biological activities of two copper(II
... attracted considerable interest due to their chelating properties [1]. The molecular structure of five-coordinated copper(II) complexes revealed an extensive variability, ranging from regular trigonal bipyramidal to regular square based pyramidal with most complexes displaying a structure that is in ...
... attracted considerable interest due to their chelating properties [1]. The molecular structure of five-coordinated copper(II) complexes revealed an extensive variability, ranging from regular trigonal bipyramidal to regular square based pyramidal with most complexes displaying a structure that is in ...
IR handout
... or acyl halide. The an aldehyde may be confirmed with C-H absorption from 2840 to 2720 cm-1. 3. the O-H or N-H absorption between 3200 and 3600 cm-1. This indicates either an alcohol, N-H containing amine or amide, or carboxylic acid. For -NH2 a doublet will be observed. 4.the C-O absorption between ...
... or acyl halide. The an aldehyde may be confirmed with C-H absorption from 2840 to 2720 cm-1. 3. the O-H or N-H absorption between 3200 and 3600 cm-1. This indicates either an alcohol, N-H containing amine or amide, or carboxylic acid. For -NH2 a doublet will be observed. 4.the C-O absorption between ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.