{Ru(trpy)(bpy)} (trpy ) 2,2
... orbital level possesses relatively high energy. The π system on DMcT2- is largely perturbed by thiol-thioamide tautomerization on protonation because the protonated DMcT2can be written in thioamide form where the five-membered ring is not aromatic (Scheme 1). The switch of aromaticity based on the t ...
... orbital level possesses relatively high energy. The π system on DMcT2- is largely perturbed by thiol-thioamide tautomerization on protonation because the protonated DMcT2can be written in thioamide form where the five-membered ring is not aromatic (Scheme 1). The switch of aromaticity based on the t ...
chemical kinetics - Berkeley City College
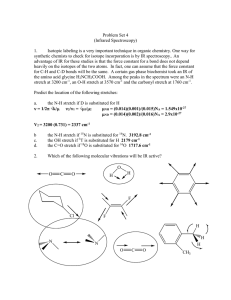
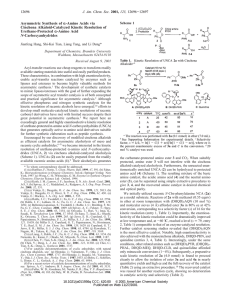
... Determining Integrated Rate Law for Reactions with More Than One Reactant For reactions that involve more than one reactant, the integrated rate law is determined for one reactant at a time. This is done by having the concentration of the limiting reactant (which rate order to be determined) conside ...
... Determining Integrated Rate Law for Reactions with More Than One Reactant For reactions that involve more than one reactant, the integrated rate law is determined for one reactant at a time. This is done by having the concentration of the limiting reactant (which rate order to be determined) conside ...
392.78 KB - KFUPM Resources v3
... 7. Substance X has a heat of vaporization of 41.8 kJ/mol at its normal boiling point (423oC). For the process X (l) X (g) at 1 atm and 423oC calculate the value of ΔSSURR. A. 0 B. 60.1 J/K mol C. 99 J/K mol D. –60.1 J/K mol E. –99 J/K mol ...
... 7. Substance X has a heat of vaporization of 41.8 kJ/mol at its normal boiling point (423oC). For the process X (l) X (g) at 1 atm and 423oC calculate the value of ΔSSURR. A. 0 B. 60.1 J/K mol C. 99 J/K mol D. –60.1 J/K mol E. –99 J/K mol ...
Amines are compounds characterized by the presence of
... secondary ammonium (R2NH2+) > tertiary ammonium (R3NH+). Quaternary ammonium salts usually exhibit the lowest solubility of the series. ...
... secondary ammonium (R2NH2+) > tertiary ammonium (R3NH+). Quaternary ammonium salts usually exhibit the lowest solubility of the series. ...
Student Review Packet
... container and allowed to reach equilibrium at 1,000 K. Determine whether the equilibrium concentration of HI(g) will be greater than, equal to, or less than the initial concentration of HI(g). Justify your answer. 2004B #1 N2(g) + 3 H2(g) 2 NH3(g) For the reaction represented above, the value of ...
... container and allowed to reach equilibrium at 1,000 K. Determine whether the equilibrium concentration of HI(g) will be greater than, equal to, or less than the initial concentration of HI(g). Justify your answer. 2004B #1 N2(g) + 3 H2(g) 2 NH3(g) For the reaction represented above, the value of ...
Document
... LEARNING CHECK The chlorine atoms can be added to either carbon 2 or 3 in this addition ...
... LEARNING CHECK The chlorine atoms can be added to either carbon 2 or 3 in this addition ...
Inorganic Chemistry 411/511
... [7 pts] Which structure is likely for BaF2 (s), rocksalt, fluorite, or sphalerite? Explain briefly. Fluorite, this is an AB2 type compound. ...
... [7 pts] Which structure is likely for BaF2 (s), rocksalt, fluorite, or sphalerite? Explain briefly. Fluorite, this is an AB2 type compound. ...
ETHER
... Has a pair of alkyl or atomic groups attached to a linking oxygen atom. Functional group is ROR Have primary, secondary, and tertiary structures ...
... Has a pair of alkyl or atomic groups attached to a linking oxygen atom. Functional group is ROR Have primary, secondary, and tertiary structures ...
Mass Spectrometry and Free Radicals MS recap Positive mode of
... Simple straight chain ketones will often fragment in “both directions” e.g. MS of 2-butanone. ...
... Simple straight chain ketones will often fragment in “both directions” e.g. MS of 2-butanone. ...
Synthesis and characterization of transition metal coordination
... essentially the same procedure. [Co(BDC)(ABZ)(H 2 O)].H 2 O is typical. To an ethanolic solution (15 mL) of Co(II) chloride (0.57 g, 2.4 mmol), a solution of BDC (0.4 g of H 2 BDC in 15 mL of 0.1 M NaOH, 2.4 mmol) was added dropwise with stirring, and then a solution of ABZ (0.362 g in 15 mL of etha ...
... essentially the same procedure. [Co(BDC)(ABZ)(H 2 O)].H 2 O is typical. To an ethanolic solution (15 mL) of Co(II) chloride (0.57 g, 2.4 mmol), a solution of BDC (0.4 g of H 2 BDC in 15 mL of 0.1 M NaOH, 2.4 mmol) was added dropwise with stirring, and then a solution of ABZ (0.362 g in 15 mL of etha ...
Dioxouranium(VI) Complexes of Some Monovalent Bidentate Schiff
... The infrared spectra of the present complexes are compared with those of corresponding ligand to determine the coordination sites of the ligands. The characteristic strong bands at 865-960 cm-1 and 785-880 cm-1 in the spectra of complexes have been assigned to νas(O=U=O) and νs(O=U=O), respectively ...
... The infrared spectra of the present complexes are compared with those of corresponding ligand to determine the coordination sites of the ligands. The characteristic strong bands at 865-960 cm-1 and 785-880 cm-1 in the spectra of complexes have been assigned to νas(O=U=O) and νs(O=U=O), respectively ...
Full Article - PDF - Brandeis University
... the carbamate-protected amino ester 3 and CO2. When suitably protected, amino ester 3 will not interfere with the cinchona alkaloid-catalyzed alcoholysis. Furthermore, the unreacted enantiomerically enriched UNCA (2) can be hydrolyzed to protected amino acid (4) (Scheme 1). The resulting mixture of ...
... the carbamate-protected amino ester 3 and CO2. When suitably protected, amino ester 3 will not interfere with the cinchona alkaloid-catalyzed alcoholysis. Furthermore, the unreacted enantiomerically enriched UNCA (2) can be hydrolyzed to protected amino acid (4) (Scheme 1). The resulting mixture of ...
experiment
... Today we will explore cobalt compounds. Co has several stable oxidation states, the most common are Co2+ and Co3+. The oxidation can be achieved by reacting Co2+ with O2 or H2O2. We will bind different ligands to the metal, some as simple as H2O, others larger organic compounds such as phenanthrene. ...
... Today we will explore cobalt compounds. Co has several stable oxidation states, the most common are Co2+ and Co3+. The oxidation can be achieved by reacting Co2+ with O2 or H2O2. We will bind different ligands to the metal, some as simple as H2O, others larger organic compounds such as phenanthrene. ...
Second Year - WordPress.com
... Dobreiner’s work led to the law of triads which states that ______ a) Atomic weight of any one element was found to be approximately the mean of the other two elements of triad. b) Atomic weight of the middle element was found to be approximately the mean of the other two elements of a triad. c) Ato ...
... Dobreiner’s work led to the law of triads which states that ______ a) Atomic weight of any one element was found to be approximately the mean of the other two elements of triad. b) Atomic weight of the middle element was found to be approximately the mean of the other two elements of a triad. c) Ato ...
SYNTHESIS, CHARACTERIZATION, ANTIMICROBIAL ACTIVITY AND CYTOTOXICITY STUDIES - METAL COMPLEXES
... field and it has been shown that many of them possess anticancer activity.[3-4]. The use of transition metal complexes with imine nitrogen as one of the donor, in catalysis has been receiving significant attention during past two decades. A wide number of different imine base ligands have been used ...
... field and it has been shown that many of them possess anticancer activity.[3-4]. The use of transition metal complexes with imine nitrogen as one of the donor, in catalysis has been receiving significant attention during past two decades. A wide number of different imine base ligands have been used ...
9 - MIT
... Attach the porphyrin to a solid support to avoid the bimolecular reaction; or, use low T, non-aqueous solvents, and py or 1-MeIm complexes, but stability is lost at - 45 °C or above. The best solution was the construction of a sterically hindered cavity for dioxygen binding to avoid the intemolecula ...
... Attach the porphyrin to a solid support to avoid the bimolecular reaction; or, use low T, non-aqueous solvents, and py or 1-MeIm complexes, but stability is lost at - 45 °C or above. The best solution was the construction of a sterically hindered cavity for dioxygen binding to avoid the intemolecula ...
alcohols03
... Sodium borohydride (NaBH4) is a safe, effective reducing agent for this reaction. Aldehydes are reduced to 1 alcohols. Ketones are reduced to 2 alcohols. Carbon-tocarbon double bonds in both these compounds are not reduced. Lithium aluminum hydride (LiAlH4) can also be used, giving higher yields, ...
... Sodium borohydride (NaBH4) is a safe, effective reducing agent for this reaction. Aldehydes are reduced to 1 alcohols. Ketones are reduced to 2 alcohols. Carbon-tocarbon double bonds in both these compounds are not reduced. Lithium aluminum hydride (LiAlH4) can also be used, giving higher yields, ...
The 9-Phenyl-9-fluorenyl Group for Nitrogen Protection in
... state. The anti product arises when the electrophile approaches from the less hindered face opposite to the Pf group. Lithium enolate has a E-geometry so it can not chelate. The electrophile approaches from the less hindered face and the product is syn (Figure 4) [24]. Figure 4. Silyl ketene acetals ...
... state. The anti product arises when the electrophile approaches from the less hindered face opposite to the Pf group. Lithium enolate has a E-geometry so it can not chelate. The electrophile approaches from the less hindered face and the product is syn (Figure 4) [24]. Figure 4. Silyl ketene acetals ...
Chapter 11, Kinetics
... concentration be at the end of that period? Ans. 0.0196 M b. How long will it take for the concentration of the solution to drop from 0.0200 M to 0.00350 M? Ans. 5.09 x 103 days (~14 yrs) c. What is the half-life for this hormone? Ans. 2.03 x 103 days (5.5 yrs) 17. In the first order decomposition o ...
... concentration be at the end of that period? Ans. 0.0196 M b. How long will it take for the concentration of the solution to drop from 0.0200 M to 0.00350 M? Ans. 5.09 x 103 days (~14 yrs) c. What is the half-life for this hormone? Ans. 2.03 x 103 days (5.5 yrs) 17. In the first order decomposition o ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.