Full-Text PDF
... for epoxidation reactions is well illustrated by some past reviews on the matter. In particular, iron-porphyrins have been the subject of extensive reviews, due to their biological relevance as part of cytochrome P450 [14,15], with their use as catalysts in epoxidation reactions being highlighted on ...
... for epoxidation reactions is well illustrated by some past reviews on the matter. In particular, iron-porphyrins have been the subject of extensive reviews, due to their biological relevance as part of cytochrome P450 [14,15], with their use as catalysts in epoxidation reactions being highlighted on ...
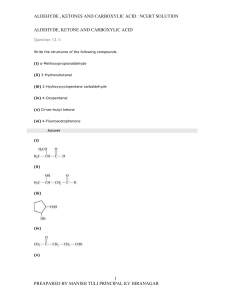
carbonyl compound group
... (v) Hemiacetal (vi) Oxime (vii) Ketal (vii) Imine (ix) 2,4-DNP-derivative (x) Schiff’s base ...
... (v) Hemiacetal (vi) Oxime (vii) Ketal (vii) Imine (ix) 2,4-DNP-derivative (x) Schiff’s base ...
(Z)-Tamoxifen and Tetrasubstituted Alkenes and Dienes via a Regio
... Skipped dienes were generated with allyl substituents (entries 3 and 4). In these cases the allyl functionality may be introduced as either the magnesium or palladium component. Dienes were prepared efficiently as demonstrated by entries 5, 6, and 7. In theses examples also, depending upon the synth ...
... Skipped dienes were generated with allyl substituents (entries 3 and 4). In these cases the allyl functionality may be introduced as either the magnesium or palladium component. Dienes were prepared efficiently as demonstrated by entries 5, 6, and 7. In theses examples also, depending upon the synth ...
Grignard knowledge: Alkyl coupling chemistry with inexpensive
... reagent. An important point to consider is OF GRIGNARD REAGENTS temporary warehousing. The result of that Grignard reagents such as methyl-, cooling is precipitation of magnesium ethyl-, propyl-, or butylmagnesium halides, Although Grignard reactions trace back salts. While this does not irreparably ...
... reagent. An important point to consider is OF GRIGNARD REAGENTS temporary warehousing. The result of that Grignard reagents such as methyl-, cooling is precipitation of magnesium ethyl-, propyl-, or butylmagnesium halides, Although Grignard reactions trace back salts. While this does not irreparably ...
Organic Chemistry - UCR Chemistry
... Unwanted Oxidation of Aldehydes. Cr(VI) reagents are powerful oxidizing agents useful for oxidizing 2° alcohols to ketones (Figure 17.005) because ketones are resistant to further oxidation. However aldehydes formed from oxidation of 1° alcohols using Cr(VI) reagents are usually further oxidized to ...
... Unwanted Oxidation of Aldehydes. Cr(VI) reagents are powerful oxidizing agents useful for oxidizing 2° alcohols to ketones (Figure 17.005) because ketones are resistant to further oxidation. However aldehydes formed from oxidation of 1° alcohols using Cr(VI) reagents are usually further oxidized to ...
Calixarene Complexes with Transition Metal, Lanthanide and
... The reactivity of CrIII in 18 and 19 is very low, even under photochemical or thermal conditions. Compound 17, however, undergoes one electron reduction leading to 20 by treatment with sodium in the presence of naphthalene in THF. Complex 20 crystallizes with THF in 1:2 molar ratio, one of the two T ...
... The reactivity of CrIII in 18 and 19 is very low, even under photochemical or thermal conditions. Compound 17, however, undergoes one electron reduction leading to 20 by treatment with sodium in the presence of naphthalene in THF. Complex 20 crystallizes with THF in 1:2 molar ratio, one of the two T ...
Q4) How the following conversions can be carried out?
... group should be unhindered. In case of secondary or tertiary alcohols, the alkyl group is hindered. As a result, elimination dominates substitution. ...
... group should be unhindered. In case of secondary or tertiary alcohols, the alkyl group is hindered. As a result, elimination dominates substitution. ...
02A naming alkane alkene alkyne 2
... • If there is only one substituent, do not use the “1”. • If there is more than one substituent, you must use all numbers, including “1”! • If there are two or more side groups, the numbering must start with a side group and then proceed in the direction that gives the lowest possible numbers to all ...
... • If there is only one substituent, do not use the “1”. • If there is more than one substituent, you must use all numbers, including “1”! • If there are two or more side groups, the numbering must start with a side group and then proceed in the direction that gives the lowest possible numbers to all ...
Full-Text PDF
... (Dublin, Ireland). Analytical HPLC (HPLC A and B) was carried out on using HPLC A: a Waters instrument comprising two solvent-delivery pumps (Waters 1525), an automatic injector (Waters 717 auto sampler), diode array wavelength detector (Waters 2487), and linear gradients of MeCN (+0.036% TFA) into ...
... (Dublin, Ireland). Analytical HPLC (HPLC A and B) was carried out on using HPLC A: a Waters instrument comprising two solvent-delivery pumps (Waters 1525), an automatic injector (Waters 717 auto sampler), diode array wavelength detector (Waters 2487), and linear gradients of MeCN (+0.036% TFA) into ...
Novel SnCl2-H2SO4 Mixed Catalyst System for Fructose
... remove a proton from a hydroxyl group in a fructose molecule, which can then be hydrolyzed to an aldehyde. The HMF is finally formed by the removal of two more water molecules from the fructose moiety by the tin (II) chloride dehydrate. This hypothetical theory therefore attribute the high HMF yield ...
... remove a proton from a hydroxyl group in a fructose molecule, which can then be hydrolyzed to an aldehyde. The HMF is finally formed by the removal of two more water molecules from the fructose moiety by the tin (II) chloride dehydrate. This hypothetical theory therefore attribute the high HMF yield ...
CfE Advanced Higher Chemistry Unit 2: Organic
... If we look at the example of H 2 (g) one of the molecular orbitals is formed by adding the mathematical functions for the two 1s orbitals that come together to form this molecule. A molecular orbital is a mathematical function describing the wave-like behaviour of an electron in a molecule. This fun ...
... If we look at the example of H 2 (g) one of the molecular orbitals is formed by adding the mathematical functions for the two 1s orbitals that come together to form this molecule. A molecular orbital is a mathematical function describing the wave-like behaviour of an electron in a molecule. This fun ...
Aldehydes and Ketones
... hemiacetals [RCH(OH)OR']. Further reaction with excess alcohol gives acetals [RCH(OR')2]. Ketones react similarly. These reactions are reversible; that is, acetals can be readily hydrolyzed by aqueous acid to their alcohol and carbonyl components. Water adds similarly to the carbonyl group of certai ...
... hemiacetals [RCH(OH)OR']. Further reaction with excess alcohol gives acetals [RCH(OR')2]. Ketones react similarly. These reactions are reversible; that is, acetals can be readily hydrolyzed by aqueous acid to their alcohol and carbonyl components. Water adds similarly to the carbonyl group of certai ...
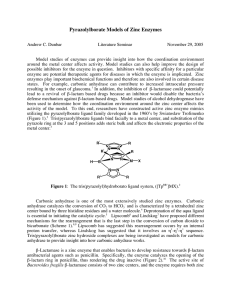
Pyrazolylborate Models of Zinc Enzymes
... enzyme are potential therapeutic agents for diseases in which the enzyme is implicated. Zinc enzymes play important biochemical functions and therefore are also involved in certain disease states. For example, carbonic anhydrase can contribute to increased intraocular pressure resulting in the onset ...
... enzyme are potential therapeutic agents for diseases in which the enzyme is implicated. Zinc enzymes play important biochemical functions and therefore are also involved in certain disease states. For example, carbonic anhydrase can contribute to increased intraocular pressure resulting in the onset ...
Solubility & Complex Ions
... A solution contains 0.25M Ni(NO3)2 and 0.25M Cu(NO3)2. Can the metal ions be separated by slowly adding Na2CO3? (Assume that for successful precipitation 99% of the metal ion must be precipitated before the other metal ion begins to precipitate, and assume no volume change on addition of sodium carb ...
... A solution contains 0.25M Ni(NO3)2 and 0.25M Cu(NO3)2. Can the metal ions be separated by slowly adding Na2CO3? (Assume that for successful precipitation 99% of the metal ion must be precipitated before the other metal ion begins to precipitate, and assume no volume change on addition of sodium carb ...
Maillard browning
... view. This may be especially important where the amino acid is very reactive and in foods where it is already in very low concentration. This would be the case for Llysine in cereals. ...
... view. This may be especially important where the amino acid is very reactive and in foods where it is already in very low concentration. This would be the case for Llysine in cereals. ...
Topic 10 SL Mark Scheme Past exam paper questions
... tertiary carbon atoms with examples. Examples of how to name non-cyclic organic compounds from structural drawings and vice-versa, with chains of up to 6 carbon atoms with one of the following functional groups: (alkane), alkene, alcohol, aldehyde, ketone, carboxylic acid and halide. Examples of com ...
... tertiary carbon atoms with examples. Examples of how to name non-cyclic organic compounds from structural drawings and vice-versa, with chains of up to 6 carbon atoms with one of the following functional groups: (alkane), alkene, alcohol, aldehyde, ketone, carboxylic acid and halide. Examples of com ...
Chapter 10 - Chemistry Solutions
... one outlined by the green box, containing five carbon atoms. This chain is numbered from right to left in order to give the hydroxyl-bearing carbon atom the lowest possible number. ...
... one outlined by the green box, containing five carbon atoms. This chain is numbered from right to left in order to give the hydroxyl-bearing carbon atom the lowest possible number. ...
Ch-6-Alcohols and phenols - Home
... Methanol and ethanol can be used as an alternative to fossil fuels as they burn very cleanly, producing only carbon dioxide and water. Ethanol is considered a renewable fuel as it can be made from renewable sources such a sugar cane and can be used as a fuel in its own right, or in mixtures with pet ...
... Methanol and ethanol can be used as an alternative to fossil fuels as they burn very cleanly, producing only carbon dioxide and water. Ethanol is considered a renewable fuel as it can be made from renewable sources such a sugar cane and can be used as a fuel in its own right, or in mixtures with pet ...
A Stable Five-Coordinate Platinum(IV) Alkyl
... transition metal complexes.1 These species containing an “opensite” in the metal coordination sphere are notoriously difficult to characterize, owing to their inherent reactivity.2 In particular, fivecoordinate Pt(IV) alkyl species have been consistently proposed as key intermediates in reductive el ...
... transition metal complexes.1 These species containing an “opensite” in the metal coordination sphere are notoriously difficult to characterize, owing to their inherent reactivity.2 In particular, fivecoordinate Pt(IV) alkyl species have been consistently proposed as key intermediates in reductive el ...
Bis-Triazinyl-Pyridines for selective extraction of americium(III
... instead of more than 100,000 years2 in the absence of any partitioning. After their removal from the spent fuels, minor actinides could be transmuted into short lived radionuclides by neutron bombardment3,4. The difficulty faced when developing partitioning processes is that spent fuels also contain ...
... instead of more than 100,000 years2 in the absence of any partitioning. After their removal from the spent fuels, minor actinides could be transmuted into short lived radionuclides by neutron bombardment3,4. The difficulty faced when developing partitioning processes is that spent fuels also contain ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.