
Ruthenium Half-Sandwich Complexes as Protein Kinase Inhibitors
... compound 58 was hydrolyzed with K2CO3 to the carboxylic acid 6 (89%) and subsequently protected as the 2-(trimethylsilyl)ethyl ester 7 by EDCI-coupling with 2-trimethylsilylethanol (62%) (Scheme 1).9 This was followed by the photochemical replacement of benzene by three acetonitrile ligands and a su ...
... compound 58 was hydrolyzed with K2CO3 to the carboxylic acid 6 (89%) and subsequently protected as the 2-(trimethylsilyl)ethyl ester 7 by EDCI-coupling with 2-trimethylsilylethanol (62%) (Scheme 1).9 This was followed by the photochemical replacement of benzene by three acetonitrile ligands and a su ...
The Carboxylic Acid Group as an Effective Director of Ortho
... metalation, providing useful ketone syntheses".11 However this situation is not universally true, since the carboxylate group from benzene aromatic systems was described in a few examples to be resistant to addition of the alkyllithium base at low temperature (i.e., 5-78 OC).12J3Furthermore, lithiat ...
... metalation, providing useful ketone syntheses".11 However this situation is not universally true, since the carboxylate group from benzene aromatic systems was described in a few examples to be resistant to addition of the alkyllithium base at low temperature (i.e., 5-78 OC).12J3Furthermore, lithiat ...
CHAPTER-7
... Ans. a) Increase in pressure increases rate of backward reaction because nP(2) >nR(1) b) Increase in pressure increases rate of forward reaction because nR(2) >nP(1) c) Increase in pressure increases rate of backward reaction because nP(2)
... Ans. a) Increase in pressure increases rate of backward reaction because nP(2) >nR(1) b) Increase in pressure increases rate of forward reaction because nR(2) >nP(1) c) Increase in pressure increases rate of backward reaction because nP(2)
Reactions of Aromatic Compounds
... • All steps are reversible, so sulfonic acid group can be removed by heating in dilute sulfuric acid. • This process is used to place deuterium in place of hydrogen on benzene ring. ...
... • All steps are reversible, so sulfonic acid group can be removed by heating in dilute sulfuric acid. • This process is used to place deuterium in place of hydrogen on benzene ring. ...
Functionalization of N2 to NH3 via direct N ≡ N bond cleavage
... presence of [LutH]+ and [Cp*2 Cr] in Heptane, Toluene and THF under normal experimental conditions, and observed a feasible situation for the reduction of nitride to ammonia (figure 7 and figures S3–S5). A comparison of all calculated thermodynamic barriers of [(Me2 N)3 W] and [(Me2 N)3 Mo] reaction ...
... presence of [LutH]+ and [Cp*2 Cr] in Heptane, Toluene and THF under normal experimental conditions, and observed a feasible situation for the reduction of nitride to ammonia (figure 7 and figures S3–S5). A comparison of all calculated thermodynamic barriers of [(Me2 N)3 W] and [(Me2 N)3 Mo] reaction ...
Chemical Reactions
... Why must chemical equations be balanced? It’s the law! Matter cannot be created or destroyed in chemical reactions. This is the law of conservation of mass. In every chemical reaction, the same mass of matter must end up in the products as started in the reactants. Balanced chemical equations show t ...
... Why must chemical equations be balanced? It’s the law! Matter cannot be created or destroyed in chemical reactions. This is the law of conservation of mass. In every chemical reaction, the same mass of matter must end up in the products as started in the reactants. Balanced chemical equations show t ...
1 THE BARTON-McCOMBIE REACTION STUART W. McCOMBIE 28
... Sarojit Sur, and Santiago Vasquez for assistance with data collection and table preparation; and to Professor Samir Zard for valuable discussions. This chapter is dedicated to the memory of Professor Derek H. R. Barton, mentor and friend. ...
... Sarojit Sur, and Santiago Vasquez for assistance with data collection and table preparation; and to Professor Samir Zard for valuable discussions. This chapter is dedicated to the memory of Professor Derek H. R. Barton, mentor and friend. ...
1.02 x 10 = 3 mol lit 3.4 x 10
... Ans: Mg(OH)2 is a weak base. NH4Cl is acidic due to hydrolysis. So neutralization takes place and dissolves. (ii) When hydrogen sulphide is passed through acidified zinc sulphate solution, white ppt of zinc sulphide is not formed. Ans: The solubility product of zinc sulphide is high and is not excee ...
... Ans: Mg(OH)2 is a weak base. NH4Cl is acidic due to hydrolysis. So neutralization takes place and dissolves. (ii) When hydrogen sulphide is passed through acidified zinc sulphate solution, white ppt of zinc sulphide is not formed. Ans: The solubility product of zinc sulphide is high and is not excee ...
Reactions involving HCl and their Evaporation
... derivatives. The t-BOC group can be removed easily from the amine using strong acids such as trifluoroacetic acid (TFA) neat or in Dichloromethane, or HCl in Dioxane. t-BOC derivatives are one of the most widely used amino protecting groups in organic synthesis. Following on from deprotection the re ...
... derivatives. The t-BOC group can be removed easily from the amine using strong acids such as trifluoroacetic acid (TFA) neat or in Dichloromethane, or HCl in Dioxane. t-BOC derivatives are one of the most widely used amino protecting groups in organic synthesis. Following on from deprotection the re ...
Chapter 20: Carboxylic Acids and Nitriles
... Most amines that have 3 different substituents on N are not resolved because the molecules interconvert by pyramidal inversion ...
... Most amines that have 3 different substituents on N are not resolved because the molecules interconvert by pyramidal inversion ...
HYBRID MULTIDENTATE PHOSPHINE
... A readily exploited characteristic of transition metal chemistry is its variation of oxidation states, which can be achieved with a wide variety of ligands and metal combinations. This allows the reactivity of a given transition metal complex to be tuned for use in, for example, catalytic processes ...
... A readily exploited characteristic of transition metal chemistry is its variation of oxidation states, which can be achieved with a wide variety of ligands and metal combinations. This allows the reactivity of a given transition metal complex to be tuned for use in, for example, catalytic processes ...
C:\SUBJECTS\SUBJECTS\Chemistry
... copper ions to form copper. This is due to the fact that A. iron is in the metallic form while dthe copper is in the ionic form B. the atomic weight of copper is greater than that of ion C. copper metal has more electrons than ion metal D. iron is an inert metal E. iron is higher in the electrochemi ...
... copper ions to form copper. This is due to the fact that A. iron is in the metallic form while dthe copper is in the ionic form B. the atomic weight of copper is greater than that of ion C. copper metal has more electrons than ion metal D. iron is an inert metal E. iron is higher in the electrochemi ...
Chapter 9 Coordination Chemistry I: Structure and Isomers
... Coordination complexes were known - although not understood in any sense - since the beginning of chemistry, e.g. Prussian blue, Aureolin, and copper vitriol. The key breakthrough occurred when Alfred Werner proposed, inter alia, that Co(III) bears six ligands in an octahedral geometry. The theory a ...
... Coordination complexes were known - although not understood in any sense - since the beginning of chemistry, e.g. Prussian blue, Aureolin, and copper vitriol. The key breakthrough occurred when Alfred Werner proposed, inter alia, that Co(III) bears six ligands in an octahedral geometry. The theory a ...
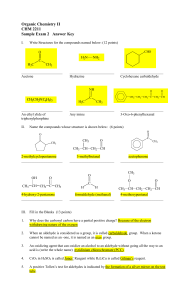
Organic Chemistry II CHM 2211 Sample Exam 2 Answer Key
... acid is (write the whole name) pyridinium chlorochromate (PCC). ...
... acid is (write the whole name) pyridinium chlorochromate (PCC). ...
Principles of Drug Action I, Spring 2004
... 7. How many different (regioisomeric and stereoisomeric) electrophilic addition products could form in the following reaction? Two regioisomers are possible by addition of OH- and Cl+ across the double bond. During addition a chiral center is created at each addition site (for each regioisomer). Thu ...
... 7. How many different (regioisomeric and stereoisomeric) electrophilic addition products could form in the following reaction? Two regioisomers are possible by addition of OH- and Cl+ across the double bond. During addition a chiral center is created at each addition site (for each regioisomer). Thu ...
A Few Things You Might Want To Know
... Mixtures can be heterogeneous or homogeneous (= solutions). They consist of substances that can be separated by physical changes (distillation, crystallization, chromatography). Substances can be either elements or compounds. Compounds can be separated into elements by chemical changes (redox reacti ...
... Mixtures can be heterogeneous or homogeneous (= solutions). They consist of substances that can be separated by physical changes (distillation, crystallization, chromatography). Substances can be either elements or compounds. Compounds can be separated into elements by chemical changes (redox reacti ...
Chemistry JAMB Past Questions
... CO is poisonous CO is readily oxidized at room temperature by air to form Co2 CO may be prepared by reducing CO2, mixed coke heated to about 1000oC CO may be prepared by heating charcoal with a limited amount of O2 CO is a good reducing agent. ...
... CO is poisonous CO is readily oxidized at room temperature by air to form Co2 CO may be prepared by reducing CO2, mixed coke heated to about 1000oC CO may be prepared by heating charcoal with a limited amount of O2 CO is a good reducing agent. ...
T_AllylCF3paperBM[5]
... smaller charges of FeCl3 (1-10%) decreases the yields dramatically. Having found optimized conditions we synthesized dimeric indanes 3 from other allyl alcohols 1 (Scheme 2). To determine the stereochemical structures of the isomers 3a-c, and 3a'-c' we have separated them by preparative TLC. Unfortu ...
... smaller charges of FeCl3 (1-10%) decreases the yields dramatically. Having found optimized conditions we synthesized dimeric indanes 3 from other allyl alcohols 1 (Scheme 2). To determine the stereochemical structures of the isomers 3a-c, and 3a'-c' we have separated them by preparative TLC. Unfortu ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.





















![T_AllylCF3paperBM[5]](http://s1.studyres.com/store/data/003584459_1-3decab572f7fca68901a941affab18ea-300x300.png)