to Dowload Part 1: PowerPoint Presentation.
... 4. Give three examples of aldehydes used for this purpose and their characteristic quality. 5. List two uses of ketones in the food industry. 6. Name a common sugar that is: a) an aldehyde and b) a ketone ...
... 4. Give three examples of aldehydes used for this purpose and their characteristic quality. 5. List two uses of ketones in the food industry. 6. Name a common sugar that is: a) an aldehyde and b) a ketone ...
Revised organic compounds containing Nitrogen
... This method is only useful for the preparation of primary nitroalkanes. With the secondary halides the yield is very low, and with tertiary halides even lower. This method has been modified by Kornblum et al for the synthesis of primary and secondary nitro-compounds. Here, the alkyl halide is reacte ...
... This method is only useful for the preparation of primary nitroalkanes. With the secondary halides the yield is very low, and with tertiary halides even lower. This method has been modified by Kornblum et al for the synthesis of primary and secondary nitro-compounds. Here, the alkyl halide is reacte ...
Atomic Structure
... Bond order is a concept in the molecular orbital theory. It depends on the number of electrons in the bonding and antibonding orbitals. Which of the following statements is true about it? The bond order (a) Cannot be a negative quantity (b) Always has an integral value (c) Can assume any value, posi ...
... Bond order is a concept in the molecular orbital theory. It depends on the number of electrons in the bonding and antibonding orbitals. Which of the following statements is true about it? The bond order (a) Cannot be a negative quantity (b) Always has an integral value (c) Can assume any value, posi ...
PROFESSOR SIR DEREK H. R. BARTON AND HETEROCYCLES
... Professor Barton's contributions to chemistry are quite enormous and influential. He has been always creating new and long-lasting fashions in chemistry, and they have contributed not only to the progress of chemistry but also to human welfare. When I met 'him for the first time at his office of th ...
... Professor Barton's contributions to chemistry are quite enormous and influential. He has been always creating new and long-lasting fashions in chemistry, and they have contributed not only to the progress of chemistry but also to human welfare. When I met 'him for the first time at his office of th ...
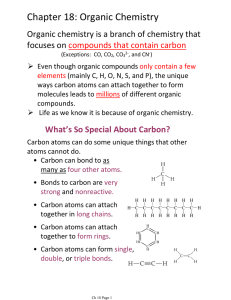
Chapter 18: Organic Chemistry
... focuses on compounds that contain carbon (Exceptions: CO, CO2, CO32-, and CN-) ...
... focuses on compounds that contain carbon (Exceptions: CO, CO2, CO32-, and CN-) ...
Chemical Reactions
... chemical reactions also have the potential to produce hazardous substances. On July 9, 1997, 400 t (4.00 × 105 kg) of plastic caught fire at a plastics manufacturing site in Hamilton, Ontario (Figure 1). The fire raged for more than three days, engulfing parts of Hamilton in thick black smoke. As a ...
... chemical reactions also have the potential to produce hazardous substances. On July 9, 1997, 400 t (4.00 × 105 kg) of plastic caught fire at a plastics manufacturing site in Hamilton, Ontario (Figure 1). The fire raged for more than three days, engulfing parts of Hamilton in thick black smoke. As a ...
Iridium Complex-Catalyzed Highly Selective Organic Synthesis
... same group. One reason may be the stability of the carbon-metal bond. In general, the carbon-metal bond in a third row transition metal complex is more stable than that in a first or second row transition metal complex. Thus, third row transition metal complexes are likely too stable to to be used a ...
... same group. One reason may be the stability of the carbon-metal bond. In general, the carbon-metal bond in a third row transition metal complex is more stable than that in a first or second row transition metal complex. Thus, third row transition metal complexes are likely too stable to to be used a ...
Ch 7 - Practice problem (Answers)
... 24. When a strong base is used in the elimination reaction of an alkyl halide the mechanism, in general, is A) E1. B) E2. C) E1 for tertiary halides, E2 for primary and secondary halides. D) E2 for tertiary halides, E1 for primary and secondary halides. Ans: B 25. Which of the following sets of cond ...
... 24. When a strong base is used in the elimination reaction of an alkyl halide the mechanism, in general, is A) E1. B) E2. C) E1 for tertiary halides, E2 for primary and secondary halides. D) E2 for tertiary halides, E1 for primary and secondary halides. Ans: B 25. Which of the following sets of cond ...
CO ORDINATION COMPOUNDS
... (i) Role of coordination compounds in biological systems: We know that photosynthesis is made possible by the presence of the chlorophyll pigment. This pigment is a coordination compound of magnesium. In the human biological system, several coordination compounds play important roles. For example, t ...
... (i) Role of coordination compounds in biological systems: We know that photosynthesis is made possible by the presence of the chlorophyll pigment. This pigment is a coordination compound of magnesium. In the human biological system, several coordination compounds play important roles. For example, t ...
2nd Nine Weeks Notes
... a. An exponent of “1” is referred to as first order. b. An exponent of “2” is referred to as second order. c. I think you get the point! B. Method of Initial Rates. 1. Again, the initial rate is the instantaneous rate at t = 0. ...
... a. An exponent of “1” is referred to as first order. b. An exponent of “2” is referred to as second order. c. I think you get the point! B. Method of Initial Rates. 1. Again, the initial rate is the instantaneous rate at t = 0. ...
Development and Applications of Complexes
... formation of clusters of catalyst molecules on the support surface as detected with atomic force microscopy (AFM). The second part of this thesis describes the development and characterization of anisotropic particle-surfaces by electrochemical sitespecific oxidation of surface-confined thiols. Reac ...
... formation of clusters of catalyst molecules on the support surface as detected with atomic force microscopy (AFM). The second part of this thesis describes the development and characterization of anisotropic particle-surfaces by electrochemical sitespecific oxidation of surface-confined thiols. Reac ...
Slide 1 / 55 Slide 2 / 55 Slide 3 / 55
... can be reversed with no net change in either system or surroundings ...
... can be reversed with no net change in either system or surroundings ...
5.2 Calculations of Enthalpy Changes (SL/HL)
... atoms. It is an endothermic process. (+103 KJ/mol for Na and +121 KJ/mol for Cl) Ionisation Energy is the energy required to make one mole of gaseous metal ions. This is also endothermic. (+500 KJ/mol for Na) Electron Affinity is the energy released when one mole of gaseous non-metal ions are produc ...
... atoms. It is an endothermic process. (+103 KJ/mol for Na and +121 KJ/mol for Cl) Ionisation Energy is the energy required to make one mole of gaseous metal ions. This is also endothermic. (+500 KJ/mol for Na) Electron Affinity is the energy released when one mole of gaseous non-metal ions are produc ...
Lectures 4-6
... Collins Oxidation (CrO3 • 2pyridine) TL 1969, 336 - CrO3 (anhydrous) + pyridine (anhydrous) ...
... Collins Oxidation (CrO3 • 2pyridine) TL 1969, 336 - CrO3 (anhydrous) + pyridine (anhydrous) ...
Equilibrium Structures Have Real Frequencies: Obtain equilibrium
... Use the HF/6-31G* model to obtain geometries for both forms of SF4. Start with C2v symmetry for the see-saw structure and C3v symmetry for the trigonal pyramid structure. Are both structures energy minima or is there only one structure? Detail your reasoning. If there is only one structure, is it th ...
... Use the HF/6-31G* model to obtain geometries for both forms of SF4. Start with C2v symmetry for the see-saw structure and C3v symmetry for the trigonal pyramid structure. Are both structures energy minima or is there only one structure? Detail your reasoning. If there is only one structure, is it th ...
CHAPTER 2 Introduction SECTION A
... Both ion and electron transfer reactions entail the transfer of charge through the interface, which can be measured as the electric current. Based on the definition of the electric double layer, Helmholtz7 proposed a simple geometric model of the interface. This model gave rise to the concept of the ...
... Both ion and electron transfer reactions entail the transfer of charge through the interface, which can be measured as the electric current. Based on the definition of the electric double layer, Helmholtz7 proposed a simple geometric model of the interface. This model gave rise to the concept of the ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.