CH 115 Exam 2 - UAB General Chemistry Supplemental Instruction
... Multiple Choice (5 percent each, no partial credit) Assume the chemical equations on this exam are NOT balanced unless stated otherwise. 1. Balance the equation and give the stoichiometric coefficient for HCl ...
... Multiple Choice (5 percent each, no partial credit) Assume the chemical equations on this exam are NOT balanced unless stated otherwise. 1. Balance the equation and give the stoichiometric coefficient for HCl ...
Chem 400 Review Chem 350 JJ.S17
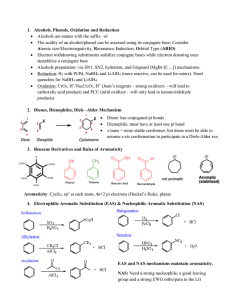
... alkyl halide (has to be 1° or 2° and sp3) Epoxide preparation is achieved with m-chloroperoxybenzoic acid (mCPBA) 6. Aldehydes, Ketones and Nucleophilic Addition Reactions Aldehydes end with the suffix –al Ketones end with the suffix –one Aldehydes are more reactive than ketones because of s ...
... alkyl halide (has to be 1° or 2° and sp3) Epoxide preparation is achieved with m-chloroperoxybenzoic acid (mCPBA) 6. Aldehydes, Ketones and Nucleophilic Addition Reactions Aldehydes end with the suffix –al Ketones end with the suffix –one Aldehydes are more reactive than ketones because of s ...
1072. A General Synthesis of Ethers.
... solution, hydrogenation of a ketal gives the same results as that of the carbonyl compound. Enolethers such as 1-methoxycyclopentene also give a mixture of alkane and saturated ether, but in a ratio different from that found for direct hydrogenation of cyclopentanone in acid methanol. This could be ...
... solution, hydrogenation of a ketal gives the same results as that of the carbonyl compound. Enolethers such as 1-methoxycyclopentene also give a mixture of alkane and saturated ether, but in a ratio different from that found for direct hydrogenation of cyclopentanone in acid methanol. This could be ...
DODH by Molybdenum Innovation Introduction DODH by Rhenium
... To elucidate the mechanism a series of experiments with variations in substrate, reductant and catalyst were carried out. To the right are shown the kinetic profiles for the standard experiment (green), less reductant (blue) and less catalyst (red), figure 5. This kinetic behaviour can be explained ...
... To elucidate the mechanism a series of experiments with variations in substrate, reductant and catalyst were carried out. To the right are shown the kinetic profiles for the standard experiment (green), less reductant (blue) and less catalyst (red), figure 5. This kinetic behaviour can be explained ...
Nomenclature - Clydebank High School
... We must add back the water which is removed in the condensation reaction. This is not very successful with water alone so we add a dilute acid to catalyse it e.g. HCl or H2SO4. (Or an alkali.) They provide H+ ions to catalyse the reaction. It is a reversible reaction ( PPA – 2) ...
... We must add back the water which is removed in the condensation reaction. This is not very successful with water alone so we add a dilute acid to catalyse it e.g. HCl or H2SO4. (Or an alkali.) They provide H+ ions to catalyse the reaction. It is a reversible reaction ( PPA – 2) ...
Chemical Synthesis Using Earth-Abundant Metal
... In the late 18th century, at the outset of the industrial revolution, the increased need for fertilizer, medicines and other commodities became so great that demand could not be satisfied by extraction from plants and animals. For the first time in history, these materials had to be made synthetical ...
... In the late 18th century, at the outset of the industrial revolution, the increased need for fertilizer, medicines and other commodities became so great that demand could not be satisfied by extraction from plants and animals. For the first time in history, these materials had to be made synthetical ...
organic chemistry - Turner Fenton Secondary School
... The two-Branches on a benzene deal: Chemists use a different method of naming benzene instead of using position numbers. The prefixes used are: ORTHO, META and PARA. ...
... The two-Branches on a benzene deal: Chemists use a different method of naming benzene instead of using position numbers. The prefixes used are: ORTHO, META and PARA. ...
Heme and Copper Oxygenases and Oxidases
... complex resembles the transition state of an oxidative addition or reductive elimination reaction. Detected by NMR spectroscopy, X-ray diffraction ...
... complex resembles the transition state of an oxidative addition or reductive elimination reaction. Detected by NMR spectroscopy, X-ray diffraction ...
1 ChE 505 WORKSHOP 1 1. Why are chemical reactions important
... What is the relationship between the initial moles of reactants and products, the moles for each of the above after some reaction time, the stoichiometric coefficients and reaction extent? ...
... What is the relationship between the initial moles of reactants and products, the moles for each of the above after some reaction time, the stoichiometric coefficients and reaction extent? ...
Carbon Compounds
... • Saturated and Unsaturated Hydrocarbons – Classification according to types of bonds – Saturated hydrocarbons (filled up) • Only single bonds it has max # of hydrogen atoms possible ...
... • Saturated and Unsaturated Hydrocarbons – Classification according to types of bonds – Saturated hydrocarbons (filled up) • Only single bonds it has max # of hydrogen atoms possible ...
Organic Chemistry Review
... What is the functional group of a carboxylic acid? What is the ending given to carboxylic acid names? What is the name of these organics? ...
... What is the functional group of a carboxylic acid? What is the ending given to carboxylic acid names? What is the name of these organics? ...
Topic 3 The chemistry of life
... 3) What are the four major macromolecules? What three elements do they all have in common? 4) What is “vitalism”? How did the synthesis of urea contribute to the falsification of vitalism? 5) Practice drawing the following: a ribose and glucose molecule as well as an amino acid and glycerol. 6) Be a ...
... 3) What are the four major macromolecules? What three elements do they all have in common? 4) What is “vitalism”? How did the synthesis of urea contribute to the falsification of vitalism? 5) Practice drawing the following: a ribose and glucose molecule as well as an amino acid and glycerol. 6) Be a ...
Esters are reduced by hydride reagents to give alcohols or aldehydes.
... Esters are reduced by hydride reagents to give alcohols or aldehydes. LiAlH4 will reduce an ester to an alcohol. Only half the equivalent of LiAlH4 is required per ester function. ...
... Esters are reduced by hydride reagents to give alcohols or aldehydes. LiAlH4 will reduce an ester to an alcohol. Only half the equivalent of LiAlH4 is required per ester function. ...
Problem Set: Empirical and Molecular Formulas
... 34.0g HCl needs 24.2 g Al(OH)3 to react completely, 12.0g Al(OH)3 is not enough 3. Ammonia, NH3, is used throughout the world as a fertilizer. To manufacture ammonia, nitrogen, N2, is combined with hydrogen, H2, in a synthesis reaction. a) Write a balanced chemical equation for the formation of ammo ...
... 34.0g HCl needs 24.2 g Al(OH)3 to react completely, 12.0g Al(OH)3 is not enough 3. Ammonia, NH3, is used throughout the world as a fertilizer. To manufacture ammonia, nitrogen, N2, is combined with hydrogen, H2, in a synthesis reaction. a) Write a balanced chemical equation for the formation of ammo ...
Handout 7
... In conclusion, all steps included in the conversion of an aldehyde or ketone to acetal or ketal via hemiacetal or hemiketal as intermediates, are reversible. Performing the reaction in large excess of an anhydrous alcohol and a small amount of an anhydrous acid will strongly favour the formation of ...
... In conclusion, all steps included in the conversion of an aldehyde or ketone to acetal or ketal via hemiacetal or hemiketal as intermediates, are reversible. Performing the reaction in large excess of an anhydrous alcohol and a small amount of an anhydrous acid will strongly favour the formation of ...
Biochemistry Worksheet
... 17. What reaction is used to breakdown polymers? Is water added or removed? How does this compare to condensation? 18. All life processes require a constant supply of ____________. Name the molecule used by cells to get energy. Give its abbreviation. 19. ATP contains what 3 functional groups covalen ...
... 17. What reaction is used to breakdown polymers? Is water added or removed? How does this compare to condensation? 18. All life processes require a constant supply of ____________. Name the molecule used by cells to get energy. Give its abbreviation. 19. ATP contains what 3 functional groups covalen ...
-23- ORGANIC CHEMISTRY A. STRUCTURE AND ISOMERISM 1
... hydrolysis (substitution) - H2O and H+ or HOAmides hydrolysis (substitution) - H2O and H+ or HOAmines (a) reaction with acid (acid-base) - e.g. HCl, CH3COOH, etc. (b) amide formation (substitution) - carboxylic acids/heat Phenols (a) reaction with strong base (acid-base) - NaOH (b) ester formation ( ...
... hydrolysis (substitution) - H2O and H+ or HOAmides hydrolysis (substitution) - H2O and H+ or HOAmines (a) reaction with acid (acid-base) - e.g. HCl, CH3COOH, etc. (b) amide formation (substitution) - carboxylic acids/heat Phenols (a) reaction with strong base (acid-base) - NaOH (b) ester formation ( ...
organic -- notes
... III. FUNCTIONAL GROUPS AND CLASSES OF ORGANIC MOLECULES A. Hydroxyl 1. Oxygen and hydrogen covalently bonded ...
... III. FUNCTIONAL GROUPS AND CLASSES OF ORGANIC MOLECULES A. Hydroxyl 1. Oxygen and hydrogen covalently bonded ...
Part B: Short Written Response - bourre-chem-11
... 18 belong. (Each one is used at least once, but may be used more often.) a) alkyl or aryl halides b) alcohols c) aldehydes d) ketones e) carboxylic acids f) esters h) hydrocarbons i) ethers ______ 1. C3H7OH ...
... 18 belong. (Each one is used at least once, but may be used more often.) a) alkyl or aryl halides b) alcohols c) aldehydes d) ketones e) carboxylic acids f) esters h) hydrocarbons i) ethers ______ 1. C3H7OH ...
MS PowerPoint - Catalysis Eprints database
... I. V. Kozhevnikov, Russ. Chem. Rev. 56 (1987) 811 M. Misono, N. Mizuno, K. Katamura, A. Kasai, Y. Konishi, K. Sakata, T. Okuhara, Y. Yoneda, Bull. Chem. Soc. Jpn. 55 (1982) 400 ...
... I. V. Kozhevnikov, Russ. Chem. Rev. 56 (1987) 811 M. Misono, N. Mizuno, K. Katamura, A. Kasai, Y. Konishi, K. Sakata, T. Okuhara, Y. Yoneda, Bull. Chem. Soc. Jpn. 55 (1982) 400 ...
Organic Chemistry Questions
... (a) What is the molecular formula for the compounds? (b) Draw the structural formulas for the four possible noncyclic isomers with this molecular formula. (c) In the presence of an appropriate catalyst, both gases add hydrogen. The hydrogenated products are identical, their molecular weight is 58. W ...
... (a) What is the molecular formula for the compounds? (b) Draw the structural formulas for the four possible noncyclic isomers with this molecular formula. (c) In the presence of an appropriate catalyst, both gases add hydrogen. The hydrogenated products are identical, their molecular weight is 58. W ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.