with answers
... when a compound (in its standard state) is formed from its constituent elements (each in their standard states). Enthalpy of reaction: The change in heat energy of the system when a chemical reaction is carried out (under constant temperature and pressure). b) Give the Gibbs-Helmholtz equation. ∆G = ...
... when a compound (in its standard state) is formed from its constituent elements (each in their standard states). Enthalpy of reaction: The change in heat energy of the system when a chemical reaction is carried out (under constant temperature and pressure). b) Give the Gibbs-Helmholtz equation. ∆G = ...
Pre DP Chemistry 2 Organic Chemistry
... 4. If more than one substituent is attached to the parent hydrocarbon, the chain is numbered in the direction that will result in the lowest possible number in the name of the compound. ...
... 4. If more than one substituent is attached to the parent hydrocarbon, the chain is numbered in the direction that will result in the lowest possible number in the name of the compound. ...
Nucleophilic Substitution Reactions of Epoxides
... Crown ethers serve as excellent phase-transfer catalysts for reactions. ...
... Crown ethers serve as excellent phase-transfer catalysts for reactions. ...
Click Here To File
... a)(i)The sum of powers of the concentration of the reactants in the rate law expression is called the order of reaction whereas the number of reacting species (atoms, ions or molecules) taking part in an elementary reaction, which must collide simultaneously in order to bring about a chemical reacti ...
... a)(i)The sum of powers of the concentration of the reactants in the rate law expression is called the order of reaction whereas the number of reacting species (atoms, ions or molecules) taking part in an elementary reaction, which must collide simultaneously in order to bring about a chemical reacti ...
Abbreviated Chapter 17 Powerpoint
... acylbenzenes to alkylbenzenes by treatment with aqueous HCl and amalgamated zinc. ...
... acylbenzenes to alkylbenzenes by treatment with aqueous HCl and amalgamated zinc. ...
File
... and halogen atom to form a hydrogen halide and an alkene. Heat and conc. KOH (alc) • CH3CH2CH2I CH3CHCH2 + HI • Consider the elimination reaction of 2-Bromobutane, what products are formed? • But-2-ene and HBr and … But-1-ene • Markovnikoff’s rule “The rich get richer, poor get poorer” • Hydrogen is ...
... and halogen atom to form a hydrogen halide and an alkene. Heat and conc. KOH (alc) • CH3CH2CH2I CH3CHCH2 + HI • Consider the elimination reaction of 2-Bromobutane, what products are formed? • But-2-ene and HBr and … But-1-ene • Markovnikoff’s rule “The rich get richer, poor get poorer” • Hydrogen is ...
This exam will consist of 30-35 multiple choice or short answer
... Briefly explain how structure of the alkyl halide substrate affects reactivity in SN2 and SN1 reactions. Why does 1-bromobutane react faster than 1-chlorobutane? Explain the two types of reactions used in this experiment to determine SN2 and SN1 reactivity. How could you tell a reaction was occurrin ...
... Briefly explain how structure of the alkyl halide substrate affects reactivity in SN2 and SN1 reactions. Why does 1-bromobutane react faster than 1-chlorobutane? Explain the two types of reactions used in this experiment to determine SN2 and SN1 reactivity. How could you tell a reaction was occurrin ...
PPT CH 11
... When an acid adds to a double bond – The H of the acid most often goes to the end of the double bond, which had more hydrogens attached initially • H-OH • H-Cl • H-Br ...
... When an acid adds to a double bond – The H of the acid most often goes to the end of the double bond, which had more hydrogens attached initially • H-OH • H-Cl • H-Br ...
Platinum Group Metal Catalysis at the End of This Century
... New reaction possibilities have been opened up by the recent application of platinum group metal homogeneous catalysts to industrial processes. In the 1960s it was widely thought that homogeneous catalysis had great potential, promising operation under mild conditions, with benefits of high activity ...
... New reaction possibilities have been opened up by the recent application of platinum group metal homogeneous catalysts to industrial processes. In the 1960s it was widely thought that homogeneous catalysis had great potential, promising operation under mild conditions, with benefits of high activity ...
Part I Power generation in fuel cells
... What is the cell reaction in the hydrogen/oxygen fuel cell? What is the cell reaction in a hydrocarbon/air fuel cell, if the hydrocarbon is methane? ...
... What is the cell reaction in the hydrogen/oxygen fuel cell? What is the cell reaction in a hydrocarbon/air fuel cell, if the hydrocarbon is methane? ...
Lecture syllabus - Linfield College
... Understanding is your goal. It is easy to discover whether or not you “understand” a concept by trying to explain it verbally to someone else in the course. If your listener grasps the concept easily from your explanation, you have proven that you understand it. If not, then most likely you need to ...
... Understanding is your goal. It is easy to discover whether or not you “understand” a concept by trying to explain it verbally to someone else in the course. If your listener grasps the concept easily from your explanation, you have proven that you understand it. If not, then most likely you need to ...
Organic Chemistry Fifth Edition
... halogen is attached to is directly attached to one other carbon. Similarly if the carbon that the halogen is attached to is directly attached to two carbons then it is a secondary alkyl halide. In tertiary alkyl halides the carbon with the halogen attached is directly attached to three other carbons ...
... halogen is attached to is directly attached to one other carbon. Similarly if the carbon that the halogen is attached to is directly attached to two carbons then it is a secondary alkyl halide. In tertiary alkyl halides the carbon with the halogen attached is directly attached to three other carbons ...
Organic Chemistry 145 CHEM
... - Bromine adds as follows; In the first step, the addition occurs mainly trans. ...
... - Bromine adds as follows; In the first step, the addition occurs mainly trans. ...
Chem 1A Final Exam – Fall 2005
... 13) Balance the following redox reactions: (Note: the chlorate ion has 3 oxygens and a -1 charge.) a) Cl2 (g) ClO3- (aq) + Cl- (aq) (chlorine dissolved in basic solution) ...
... 13) Balance the following redox reactions: (Note: the chlorate ion has 3 oxygens and a -1 charge.) a) Cl2 (g) ClO3- (aq) + Cl- (aq) (chlorine dissolved in basic solution) ...
SAMPLE TEST PAPER KPS CHEMISTRY
... Q07. What prompted Bartlet to the discovery of noble gas compounds? Q08. Carboxylic acids cannot give the properties of carbonyl group. Explain, why? Q09. a) Explain the observed Kb order: Et2 NH Et3 N Et NH2 in aqueous solution. b) Diazonium salts of aromatic amines are more stable than that of ...
... Q07. What prompted Bartlet to the discovery of noble gas compounds? Q08. Carboxylic acids cannot give the properties of carbonyl group. Explain, why? Q09. a) Explain the observed Kb order: Et2 NH Et3 N Et NH2 in aqueous solution. b) Diazonium salts of aromatic amines are more stable than that of ...
Unit 1 Chemistry
... Amino acids have two forms an L form and a D form but only the L form is found in natural proteins, what does this tell us? The presence of a creator in the formation of the proteins and also ...
... Amino acids have two forms an L form and a D form but only the L form is found in natural proteins, what does this tell us? The presence of a creator in the formation of the proteins and also ...
File
... Carbons in an organic molecule are classified based on the number of other carbons they are attached to Primary Carbon, 1°, = carbon attached to one other carbon Secondary Carbon, 2°, = carbon attached to 2 other carbons Tertiary Carbon, 3°, = carbon attached to 3 other carbons Quaternary Carbon, 4° ...
... Carbons in an organic molecule are classified based on the number of other carbons they are attached to Primary Carbon, 1°, = carbon attached to one other carbon Secondary Carbon, 2°, = carbon attached to 2 other carbons Tertiary Carbon, 3°, = carbon attached to 3 other carbons Quaternary Carbon, 4° ...
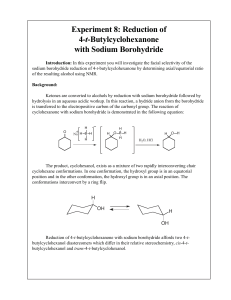
Experiment 8: Reduction of 4-t-Butylcyclohexanone with Sodium
... liquids separate into two layers or “phases” with the more dense liquid on the bottom. As a general rule of thumb, if the organic solvent is halogenated (dichloromethane) it will be more dense than water and thus on the bottom. If it is non-halogenated (ether, hexane, ethyl acetate) it will be less ...
... liquids separate into two layers or “phases” with the more dense liquid on the bottom. As a general rule of thumb, if the organic solvent is halogenated (dichloromethane) it will be more dense than water and thus on the bottom. If it is non-halogenated (ether, hexane, ethyl acetate) it will be less ...
Enantioselective Homogeneous Hydrogenation of Monosubstituted
... that none of the 9 ligands gave more than 24±27% ee in our attempts to hydrogenate 1±6. This compares well to the methyl ester of piperazine-2-carboxylic acid which gave an ee of only 3.6% [6]. It remains to be seen if the corresponding t-butyl esters and amides of pyridines 1 and 2 give much higher ...
... that none of the 9 ligands gave more than 24±27% ee in our attempts to hydrogenate 1±6. This compares well to the methyl ester of piperazine-2-carboxylic acid which gave an ee of only 3.6% [6]. It remains to be seen if the corresponding t-butyl esters and amides of pyridines 1 and 2 give much higher ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.