Lecture notes for chapter 7
... The high stability of a cyclobutadiene coordinated to a metal arises from the mixing of the 4 electrons on the cyclobutadiene with one of the metal d orbitals that has 2 e- and the right symmetry to mix in and form a 6 electron aromatic system! This is shown on the MO diagram on the next page. N ...
... The high stability of a cyclobutadiene coordinated to a metal arises from the mixing of the 4 electrons on the cyclobutadiene with one of the metal d orbitals that has 2 e- and the right symmetry to mix in and form a 6 electron aromatic system! This is shown on the MO diagram on the next page. N ...
The use of conductivity measurements in organic solvents for the
... “For several years before 1970 my research group had been investigating transition metal complexes of heterocyclic ligands, particularly from the viewpoint of their possible use as analytical reagents. As so often happens, although this aspect proved to be disappointing another area became of intere ...
... “For several years before 1970 my research group had been investigating transition metal complexes of heterocyclic ligands, particularly from the viewpoint of their possible use as analytical reagents. As so often happens, although this aspect proved to be disappointing another area became of intere ...
Chapter 20 reactions of carbonyls
... • To determine what carbonyl and Grignard components are needed to prepare a given compound, follow these two steps: ...
... • To determine what carbonyl and Grignard components are needed to prepare a given compound, follow these two steps: ...
Chapter 4: Carbon and Molecular Diversity: KEY
... Vocabulary and Study Questions 1) List the six major elements that are components of organic molecules. C, H, N, O, P, S 2)What is tetravalence? Carbon has 4 valence electrons b) What effect does it have on carbon? Each C atom can act as an intersection point from which a molecule can branch off in ...
... Vocabulary and Study Questions 1) List the six major elements that are components of organic molecules. C, H, N, O, P, S 2)What is tetravalence? Carbon has 4 valence electrons b) What effect does it have on carbon? Each C atom can act as an intersection point from which a molecule can branch off in ...
Carbon and Hydrocarbons
... 1) Different arrangement of elements gives different properties 2) Isomers – compounds with same chemical formula but different shape Ex. C2H6O is both ethanol and dimethyl ether ...
... 1) Different arrangement of elements gives different properties 2) Isomers – compounds with same chemical formula but different shape Ex. C2H6O is both ethanol and dimethyl ether ...
Word - chemmybear.com
... State the # of atoms and # of elements in a molecule. Draw isomers of molecules and recognize isomers. Know the bond angle of H-C-H bond. State the bonding capacity of C, H, O, Cl, Br, and I. Write the formula of a hydrocarbon, given its name and vice versa. List the names and symbols of ...
... State the # of atoms and # of elements in a molecule. Draw isomers of molecules and recognize isomers. Know the bond angle of H-C-H bond. State the bonding capacity of C, H, O, Cl, Br, and I. Write the formula of a hydrocarbon, given its name and vice versa. List the names and symbols of ...
Alcohol oxidation
... Ozonolysis is the cleavage of an alkene or alkyne with ozone to form organic compounds in which the multiple carbon–carbon bond has been replaced by a double bond to oxygen. The outcome of the reaction depends on the type of multiple bond being oxidized and the workup conditions. Ozonolysis of alken ...
... Ozonolysis is the cleavage of an alkene or alkyne with ozone to form organic compounds in which the multiple carbon–carbon bond has been replaced by a double bond to oxygen. The outcome of the reaction depends on the type of multiple bond being oxidized and the workup conditions. Ozonolysis of alken ...
chapter19
... Aldehyde hydrate is oxidized to a carboxylic acid by usual reagents for alcohols ...
... Aldehyde hydrate is oxidized to a carboxylic acid by usual reagents for alcohols ...
... is advantageous over the chemical ones, as they ensure the stereo-selective obtaining of the product. Although it has been shown that their chemical synthesis was well-developed, the method still employs toxic and expensive compounds to yield α and β-configuration products via several steps. Hence, ...
Chapter 25 The Chemistry of Life: Organic Chemistry 25.1 Some
... Branched-chain hydrocarbons are possible for alkanes with four or more C atoms. Structures with different branches can be written for the same formula: ...
... Branched-chain hydrocarbons are possible for alkanes with four or more C atoms. Structures with different branches can be written for the same formula: ...
TRANSITION METALS
... iron (II). The intense purple colour disappears as the Mn 2+ ion is formed as per the equation above and so acts as its own indicator. The choice of acid used in redox titrations using MnO4- is very important. Consider the redox potentials below; ...
... iron (II). The intense purple colour disappears as the Mn 2+ ion is formed as per the equation above and so acts as its own indicator. The choice of acid used in redox titrations using MnO4- is very important. Consider the redox potentials below; ...
CHEM 203 Topics Discussed on Nov. 20 Principle: protonation of
... Electrophilic character of the above reagents and facile reaction thereof with nucleophiles Principle: the above reagents rely on the nucleophilic properties of the OH group to achieve conversion of alcohols into alkyl halides Principle: only primary and secondary alcohols are sufficiently nucleophi ...
... Electrophilic character of the above reagents and facile reaction thereof with nucleophiles Principle: the above reagents rely on the nucleophilic properties of the OH group to achieve conversion of alcohols into alkyl halides Principle: only primary and secondary alcohols are sufficiently nucleophi ...
Organic Tutorial 1st Year MT03
... Peter Sykes,“A Guidebook to Mechanism in Organic Chemistry”, and Eames & Peach “Stereochemistry at a Glance”. Notes and Questions a) Summary on not more than 6 sides. This should outline the possible mechanisms and the evidence on which they are based, in particular the evidence for inversion during ...
... Peter Sykes,“A Guidebook to Mechanism in Organic Chemistry”, and Eames & Peach “Stereochemistry at a Glance”. Notes and Questions a) Summary on not more than 6 sides. This should outline the possible mechanisms and the evidence on which they are based, in particular the evidence for inversion during ...
1. Functional groups contribute to the molecular diversity of life
... 1. Functional groups contribute to the molecular diversity of life • The components of organic molecules that are most commonly involved in chemical reactions are known as functional groups. – Functional groups are attachments that replace one or more hydrogen atoms to the carbon skeleton of the hy ...
... 1. Functional groups contribute to the molecular diversity of life • The components of organic molecules that are most commonly involved in chemical reactions are known as functional groups. – Functional groups are attachments that replace one or more hydrogen atoms to the carbon skeleton of the hy ...
Functional Groups
... 1. Functional groups contribute to the molecular diversity of life • The components of organic molecules that are most commonly involved in chemical reactions are known as functional groups. • Functional groups are attachments that replace one or more hydrogen atoms to the carbon skeleton of the hy ...
... 1. Functional groups contribute to the molecular diversity of life • The components of organic molecules that are most commonly involved in chemical reactions are known as functional groups. • Functional groups are attachments that replace one or more hydrogen atoms to the carbon skeleton of the hy ...
functional groups
... • There are six functional groups that are most important to the chemistry of life: hydroxyl, carbonyl, carboxyl, amino, sulfhydryl, and phosphate groups. • All are hydrophilic and increase solubility of organic compounds in water. ...
... • There are six functional groups that are most important to the chemistry of life: hydroxyl, carbonyl, carboxyl, amino, sulfhydryl, and phosphate groups. • All are hydrophilic and increase solubility of organic compounds in water. ...
functional groups 1. PPT
... propellants, Teflon (polymer), Brominated compounds (Fire retardant clothing) -many of these compounds are now banned from use for health and environmental reasons. ...
... propellants, Teflon (polymer), Brominated compounds (Fire retardant clothing) -many of these compounds are now banned from use for health and environmental reasons. ...
Organic Chemistry-II
... (molecular weight = 60). Deduce the structures of A, B, C, D and E. Ans5. A = CH3CH2CH2COOCH2CH3 (ethyl butanoate), B = CH3CH2OH (ethyl alcohol), C = CH3CH2CH2CH2OH (1-butanol), D = CH3CHO (acetaldehyde) E = CH3CH = CHCHO (crotonaldehyde) and F = CH3COOH (acetic acid). The reactions may be given as ...
... (molecular weight = 60). Deduce the structures of A, B, C, D and E. Ans5. A = CH3CH2CH2COOCH2CH3 (ethyl butanoate), B = CH3CH2OH (ethyl alcohol), C = CH3CH2CH2CH2OH (1-butanol), D = CH3CHO (acetaldehyde) E = CH3CH = CHCHO (crotonaldehyde) and F = CH3COOH (acetic acid). The reactions may be given as ...
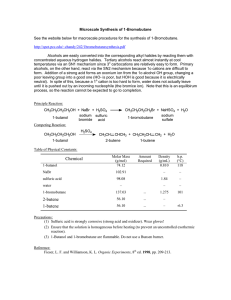
Synthesis of 1
... http://spot.pcc.edu/~chandy/242/1bromobutanesynthesis.pdf Alcohols are easily converted into the corresponding alkyl halides by reacting them with concentrated aqueous hydrogen halides. Tertiary alcohols react almost instantly at cool temperatures via an SN1 mechanism since 3o carbocations are relat ...
... http://spot.pcc.edu/~chandy/242/1bromobutanesynthesis.pdf Alcohols are easily converted into the corresponding alkyl halides by reacting them with concentrated aqueous hydrogen halides. Tertiary alcohols react almost instantly at cool temperatures via an SN1 mechanism since 3o carbocations are relat ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.