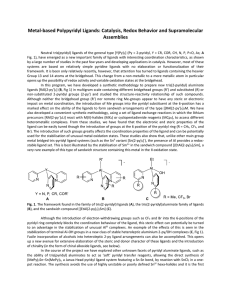
Thermo Practice Test
... Which one of the following statements best describes the relationship between G and temperature? A) G is independent of T; B) G varies with T; C) G is a linear function of T; D) G usually decreases with T. Hydrogen bromide gas and chlorine gas react to produce hydrogen chloride gas and liquid ...
... Which one of the following statements best describes the relationship between G and temperature? A) G is independent of T; B) G varies with T; C) G is a linear function of T; D) G usually decreases with T. Hydrogen bromide gas and chlorine gas react to produce hydrogen chloride gas and liquid ...
supplemenatary material - Royal Society of Chemistry
... direct contact of the solution with the steel. The solution was magnetically stirred and the temperature was controlled by a water bath circulating through an external jacket. The pressure of the autoclave was kept constant by means of a regulator, connected to a syn-gas reservoir. The evolution of ...
... direct contact of the solution with the steel. The solution was magnetically stirred and the temperature was controlled by a water bath circulating through an external jacket. The pressure of the autoclave was kept constant by means of a regulator, connected to a syn-gas reservoir. The evolution of ...
File - Mr. Heff`s Class
... - Molecular formula of benzene, C6H6, is a based on its percent composition and molar mass. - Melting point = 5.5 oC, boiling point = 80.1 oC which is comparable to the boiling of cyclohexane (81.4 0C). - Non-polar molecule and is soluble only in nonpolar solvents. - Benzene has hybrid bonds. - Benz ...
... - Molecular formula of benzene, C6H6, is a based on its percent composition and molar mass. - Melting point = 5.5 oC, boiling point = 80.1 oC which is comparable to the boiling of cyclohexane (81.4 0C). - Non-polar molecule and is soluble only in nonpolar solvents. - Benzene has hybrid bonds. - Benz ...
Organic Compound *Definition: Alcohol is organic compound in
... 'tertiary' (3°), based upon the __________ the C-OH carbon is bonded to. A primary (1°) alcohol is one in which the carbon atom with the OH group is attached to __________. Its general formula is __________. A secondary (2°) alcohol is one in which the carbon atom (in red) with the OH group is attac ...
... 'tertiary' (3°), based upon the __________ the C-OH carbon is bonded to. A primary (1°) alcohol is one in which the carbon atom with the OH group is attached to __________. Its general formula is __________. A secondary (2°) alcohol is one in which the carbon atom (in red) with the OH group is attac ...
Lecture5
... Watson’s exchange reaction between a coordinated methyl group and free methane, via σ bond metathesis, discovered by 13C isotope labeling of the methane carbon. ...
... Watson’s exchange reaction between a coordinated methyl group and free methane, via σ bond metathesis, discovered by 13C isotope labeling of the methane carbon. ...
Chapter 22/23-Organic Chemistry
... reactions(if there are more then one possible set of products name at least two): a. Pentane is cracked b. Heptane is cracked c. Propane and butane undergo a reforming reaction d. Benzene burns in air ...
... reactions(if there are more then one possible set of products name at least two): a. Pentane is cracked b. Heptane is cracked c. Propane and butane undergo a reforming reaction d. Benzene burns in air ...
UNIVERSITY OF CAMBRIDGE INTERNATIONAL
... two cations, one of which is Fe2+, one anion which is SO42–, and water of crystallisation. (a) The identity of the second cation was determined by the following test. Solid Mohr’s salt was heated with solid sodium hydroxide and a colourless gas was evolved. The gas readily dissolved in water giving ...
... two cations, one of which is Fe2+, one anion which is SO42–, and water of crystallisation. (a) The identity of the second cation was determined by the following test. Solid Mohr’s salt was heated with solid sodium hydroxide and a colourless gas was evolved. The gas readily dissolved in water giving ...
CHEM 2412
... Nomenclature and drawing of alkynes; Physical properties of alkynes; Hybridization and bond lengths, scharacter; Acidity of terminal alkynes; Acetylide formation and reactions with alkyl halides and carbonyl compounds; Elimination reactions used to form alkynes (terminal/internal isomerization); Add ...
... Nomenclature and drawing of alkynes; Physical properties of alkynes; Hybridization and bond lengths, scharacter; Acidity of terminal alkynes; Acetylide formation and reactions with alkyl halides and carbonyl compounds; Elimination reactions used to form alkynes (terminal/internal isomerization); Add ...
FUNCTIONAL GROUPS ORGANIC COMPOUNDS CONTAINING
... BSc (Ind. Chem.)(UTM), MSc (Chem)(UTM), PhD (Chem)(UTM), A.M.I.C Senior Lecturer, Department of Biotechnology and Medical Engineering, Faculty of Biosciences and Medical Engineering, ...
... BSc (Ind. Chem.)(UTM), MSc (Chem)(UTM), PhD (Chem)(UTM), A.M.I.C Senior Lecturer, Department of Biotechnology and Medical Engineering, Faculty of Biosciences and Medical Engineering, ...
Electrochemical oxidation of cinnamic acid using stainless steel
... The product was analysed by TLC technique, UV and IR spectral studies and identified as 1,4diphenylbut- 1,3-diene. This work paves a humble way of opening up a new area wherein other electro analytical parameters like polarography, cyclic voltammetry, electrode variation, temperature variation, solv ...
... The product was analysed by TLC technique, UV and IR spectral studies and identified as 1,4diphenylbut- 1,3-diene. This work paves a humble way of opening up a new area wherein other electro analytical parameters like polarography, cyclic voltammetry, electrode variation, temperature variation, solv ...
Chemical reactions cause chemical changes. They involve the
... DECOMPOSITION REACTION: In a decomposition reaction a more complex substance breaks down into simpler parts. One reactant yields 2 or more products. For example, water can be broken down into hydrogen gas and oxygen gas. The chemical equation for this decomposition reaction looks like: reactant pr ...
... DECOMPOSITION REACTION: In a decomposition reaction a more complex substance breaks down into simpler parts. One reactant yields 2 or more products. For example, water can be broken down into hydrogen gas and oxygen gas. The chemical equation for this decomposition reaction looks like: reactant pr ...
Functional Group Handout
... II. Functional Group with Carbon Singly Bonded To An Electronegative Element A. Alkyl Halides: Alkyl halides contain a halogen atom bonded to an sp3 carbon. They may be 1°, 2° or 3° depending on the substitution of the carbon atom bonded to the halogen. Vinyl halides are organic compounds which cont ...
... II. Functional Group with Carbon Singly Bonded To An Electronegative Element A. Alkyl Halides: Alkyl halides contain a halogen atom bonded to an sp3 carbon. They may be 1°, 2° or 3° depending on the substitution of the carbon atom bonded to the halogen. Vinyl halides are organic compounds which cont ...
Alkanes
... Carboxylic acids are _____ because they ionize slightly in solution to give a carboxylate ion and a _________ ion. ...
... Carboxylic acids are _____ because they ionize slightly in solution to give a carboxylate ion and a _________ ion. ...
Chapter 14 Notes
... • The carbonyl group is moderately polar, but it doesn’t have any hydrogen atoms attached, so it cannot hydrogen bond between molecules. ...
... • The carbonyl group is moderately polar, but it doesn’t have any hydrogen atoms attached, so it cannot hydrogen bond between molecules. ...
NAT 5 Unit 2 Natures Chem Booklet 1 Fuels
... uses transition metals (like Platinum or Rhodium) as catalysts. These catalysts convert pollutant gases such as carbon monoxide and nitrogen oxides to less harmful gases such as carbon dioxide and nitrogen. ...
... uses transition metals (like Platinum or Rhodium) as catalysts. These catalysts convert pollutant gases such as carbon monoxide and nitrogen oxides to less harmful gases such as carbon dioxide and nitrogen. ...
Applications of trivalent and pentavalent tantalum in organic synthesis
... yields the E-olefin. Low valent tantalum produced from TaC15-Zn which is much faster than analogues NbC15-Zn for complexation with acetylene after basic hydrolysis with H20/(NaOH) furnished the protonated olefin in good yields with more than 99% Z selectivity (Scheme x).~' It is observed that in the ...
... yields the E-olefin. Low valent tantalum produced from TaC15-Zn which is much faster than analogues NbC15-Zn for complexation with acetylene after basic hydrolysis with H20/(NaOH) furnished the protonated olefin in good yields with more than 99% Z selectivity (Scheme x).~' It is observed that in the ...
Reactions of Alkenes and Alkynes
... • Treatment of halohydrin with base deprotonates OH • O- nucleophile reacts with C-Cl electrophile substituting CO bond for C-Cl bond • Cl- eliminated yielding the epoxide ...
... • Treatment of halohydrin with base deprotonates OH • O- nucleophile reacts with C-Cl electrophile substituting CO bond for C-Cl bond • Cl- eliminated yielding the epoxide ...
Carbon and the Molecular Diversity of Life
... many of cell’s organic molecules have regions consisting of only C and H (e.g., tails of fat molecules) ...
... many of cell’s organic molecules have regions consisting of only C and H (e.g., tails of fat molecules) ...
Chapter 13
... • This makes low molecular weight alcohols highly soluble in water. • Hydrogen bonding in a water-methanol solution: ...
... • This makes low molecular weight alcohols highly soluble in water. • Hydrogen bonding in a water-methanol solution: ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.