Prelab Assignment: The lodination of Acetone
... the reaction will give you information about the order of the reaction with respect to H+. Repeat the experiment with this mixture to establish the time of reaction to within 15 seconds, again making sure that the temperature is within about a degree of that observed previously. From the rate you de ...
... the reaction will give you information about the order of the reaction with respect to H+. Repeat the experiment with this mixture to establish the time of reaction to within 15 seconds, again making sure that the temperature is within about a degree of that observed previously. From the rate you de ...
PowerPoint Presentation - Chem 101/lecture 1-2
... sp Hybridization • Promote one 2s electron into the vacant p-orbital. • Combine (mix) the 2s and 2px orbitals to give two hybrid orbitals of equivalent energy • The 2py and 2pz orbital are unaltered. E ...
... sp Hybridization • Promote one 2s electron into the vacant p-orbital. • Combine (mix) the 2s and 2px orbitals to give two hybrid orbitals of equivalent energy • The 2py and 2pz orbital are unaltered. E ...
and Square-Grooved Networks to Tubular Assemblies in
... elliptical channels run along the a axis, due to the grooves facing each other as in 1, that do not exceed 3 Å in their narrowest parts and are thus too small for any practical use. The framework is compact, as shown by its KPI of 0.77. The coordinated and free water molecules form an intricate net ...
... elliptical channels run along the a axis, due to the grooves facing each other as in 1, that do not exceed 3 Å in their narrowest parts and are thus too small for any practical use. The framework is compact, as shown by its KPI of 0.77. The coordinated and free water molecules form an intricate net ...
Carbon and its Compounds Summary Study of the compounds of
... 'R' can be a 'H' atom or an alkyl group attached to an aldehdye group (CHO). Question (9): What are homologous series? Write a short note. Answer: The extremely large numbers of carbon compounds discovered so far are better and more systematically studied by classifying them into ...
... 'R' can be a 'H' atom or an alkyl group attached to an aldehdye group (CHO). Question (9): What are homologous series? Write a short note. Answer: The extremely large numbers of carbon compounds discovered so far are better and more systematically studied by classifying them into ...
Supplementary Data - Royal Society of Chemistry
... Bruker 300 MHz spectrophotometer and Perkin Elmer 60 MHz. GC analysis was performed on Shimazdu GC-14B using packed column SE-30. Pyrrole and 2,6-dichlorobenzaldehyde, Cyclohexene and cyclooctene were obtained from Acros, USA. Styrene and p-chlorostyrene, 1-methylimidazole were obtained from Fluka C ...
... Bruker 300 MHz spectrophotometer and Perkin Elmer 60 MHz. GC analysis was performed on Shimazdu GC-14B using packed column SE-30. Pyrrole and 2,6-dichlorobenzaldehyde, Cyclohexene and cyclooctene were obtained from Acros, USA. Styrene and p-chlorostyrene, 1-methylimidazole were obtained from Fluka C ...
CH 3
... acid in a layer of hexane, which floats on the water. The polymer forms at the interface of the two layers, and a long string can be slowly pulled out. ...
... acid in a layer of hexane, which floats on the water. The polymer forms at the interface of the two layers, and a long string can be slowly pulled out. ...
Nucleophilic Aromatic Substitution, General Corrected Mechanism
... Nucleophilic aromatic substitution in electron-deficient arenas, particularly nitroarenes is an efficient tool in synthesis and manufacturing of pharmaceuticals [1-3]. It is therefore of crucial importance to know how exactly these reactions proceed. For many years it was considered that these react ...
... Nucleophilic aromatic substitution in electron-deficient arenas, particularly nitroarenes is an efficient tool in synthesis and manufacturing of pharmaceuticals [1-3]. It is therefore of crucial importance to know how exactly these reactions proceed. For many years it was considered that these react ...
Answers - Benjamin
... d The key feature of the i.r. spectrum is the change at ca 3000 cm–1 indicating the loss of a phenolic hydroxyl and/or carboxylic acid group. The n.m.r. spectra show the loss of a carboxylic acid –OH group, and the gain of a methyl group. The CH3 group is in an environment different to that in aspir ...
... d The key feature of the i.r. spectrum is the change at ca 3000 cm–1 indicating the loss of a phenolic hydroxyl and/or carboxylic acid group. The n.m.r. spectra show the loss of a carboxylic acid –OH group, and the gain of a methyl group. The CH3 group is in an environment different to that in aspir ...
Chemistry 212 — Fall Semester 1996 Examination #2
... Formic acid, HCOOH, also known as _____methanoic acid__________ in the IUPAC nomenclature, is the simplest carboxylic acid. Considering HCOOH, let us review a few properties of carboxylic acids. (a) Boiling Points. The molecular mass of HCOOH is 46 and very close to the molecular mass of 44 of propa ...
... Formic acid, HCOOH, also known as _____methanoic acid__________ in the IUPAC nomenclature, is the simplest carboxylic acid. Considering HCOOH, let us review a few properties of carboxylic acids. (a) Boiling Points. The molecular mass of HCOOH is 46 and very close to the molecular mass of 44 of propa ...
20.4 Acid-Base Properties of Carboxylic Acids
... • There are four main types of reactions: 1. Reactions at the carbonyl group 2. Reactions at the carboxylate oxygen 3. Loss of the carboxy group as CO2 (decarboxylation) 4. Reactions involving the a-carbon (Ch 22) ...
... • There are four main types of reactions: 1. Reactions at the carbonyl group 2. Reactions at the carboxylate oxygen 3. Loss of the carboxy group as CO2 (decarboxylation) 4. Reactions involving the a-carbon (Ch 22) ...
13-4 Ligands in Organometallic Chemistry
... 13-1 Historical Background Organometallic Compound Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Organometallic chemistry combines aspects of inorganic chemistry and organic chemistry. Organometallic compounds find practical use in stoichio ...
... 13-1 Historical Background Organometallic Compound Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Organometallic chemistry combines aspects of inorganic chemistry and organic chemistry. Organometallic compounds find practical use in stoichio ...
DCY1B - Manonmaniam Sundaranar University
... steroids. This catalyst transfers the hydrogen atoms specifically to the 'cis' positions. (ii) It is an ideal catalyst used for catalysing hydrogenations at room temperature and pressure. (iii) Wilkinson's catalyst is more important in pharmaceutical and petrochemical industries for making specific ...
... steroids. This catalyst transfers the hydrogen atoms specifically to the 'cis' positions. (ii) It is an ideal catalyst used for catalysing hydrogenations at room temperature and pressure. (iii) Wilkinson's catalyst is more important in pharmaceutical and petrochemical industries for making specific ...
Enolate Chemistry - Institut für Organische Chemie
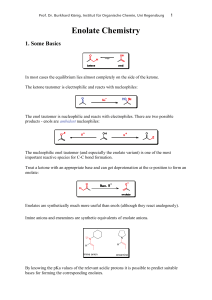
... So far we have discussed three types of enolates which sometimes give high levels of simple diastereoselectivity (syn or anti product). In most cases the relative stereocontrol is determined by the geometry of the enolate (cyclic T.S. with boron or lithium enolates). To control the absolute stereoch ...
... So far we have discussed three types of enolates which sometimes give high levels of simple diastereoselectivity (syn or anti product). In most cases the relative stereocontrol is determined by the geometry of the enolate (cyclic T.S. with boron or lithium enolates). To control the absolute stereoch ...
Alcohols, Phenols and Ethers
... Increasing order of acidity is ethanol < water < phenol. The phenoxide ion obtained after the removal of a proton is stabilised by resonance whereas the ethoxide ion obtained after the removal of a proton is destabilised by ‘+I’ effect of —C2H5 group. Therefore phenol is stronger acid than ethanol. ...
... Increasing order of acidity is ethanol < water < phenol. The phenoxide ion obtained after the removal of a proton is stabilised by resonance whereas the ethoxide ion obtained after the removal of a proton is destabilised by ‘+I’ effect of —C2H5 group. Therefore phenol is stronger acid than ethanol. ...
Chemistry
... Dinitrogen: preparation- from (NH4)2Cr2O7, laboratory method from NH4Cl, properties and uses. Compounds of nitrogen: ammonia – manufacture by Haber’s process, properties – basic character, reaction with ZnSO4 and Cu2+ ion. Nitric acid – manufacture by Ostwald’s process , laboratory method – from Na ...
... Dinitrogen: preparation- from (NH4)2Cr2O7, laboratory method from NH4Cl, properties and uses. Compounds of nitrogen: ammonia – manufacture by Haber’s process, properties – basic character, reaction with ZnSO4 and Cu2+ ion. Nitric acid – manufacture by Ostwald’s process , laboratory method – from Na ...
- Mendeley Data
... ketone or aldehyde are replaced by an imine group due to simple one-pot condensation synthesis in an alcoholic solvent. For more than a century, metal complexes of the Schiff base ligands have been rigorously studied and used in different fields including biological research [1-6], chemical analysis ...
... ketone or aldehyde are replaced by an imine group due to simple one-pot condensation synthesis in an alcoholic solvent. For more than a century, metal complexes of the Schiff base ligands have been rigorously studied and used in different fields including biological research [1-6], chemical analysis ...
ch04 by Dr. Dina
... “Straight-chain” alkanes have a zig-zag orientation when they are in their most straight orientation ...
... “Straight-chain” alkanes have a zig-zag orientation when they are in their most straight orientation ...
Large coordination complexes: synthesis, characterisation and
... presence of two methyl groups remote from the coordination centre. Their coordination properties would be expected to be identical, the only difference being their molecular weight, 28 units greater for 2 than for 1. We will call these two ligands pseudoisotopic molecules, defined as two species whi ...
... presence of two methyl groups remote from the coordination centre. Their coordination properties would be expected to be identical, the only difference being their molecular weight, 28 units greater for 2 than for 1. We will call these two ligands pseudoisotopic molecules, defined as two species whi ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.