Haloalkanes and Haloarenes
... The reaction between CH3Cl and hydroxide ion to yield methanol and chloride ion follows a second order kinetics, i.e., the rate depends upon the concentration of both the reactants. ...
... The reaction between CH3Cl and hydroxide ion to yield methanol and chloride ion follows a second order kinetics, i.e., the rate depends upon the concentration of both the reactants. ...
IB2 SL CHEMISTRY Name: …………………………… Topic 10
... Allow double bonds on arene in alternate positions, or allow delocalized representation (of pi electrons). ...
... Allow double bonds on arene in alternate positions, or allow delocalized representation (of pi electrons). ...
Carbon - HCC Learning Web
... A few number of chemical groups are key to the functioning of biological molecules • The distinctive properties of an organic molecule depend not only on the arrangement of its carbon skeleton but also on the chemical groups attached to that skeleton. • If we start with hydrocarbons as the simplest ...
... A few number of chemical groups are key to the functioning of biological molecules • The distinctive properties of an organic molecule depend not only on the arrangement of its carbon skeleton but also on the chemical groups attached to that skeleton. • If we start with hydrocarbons as the simplest ...
AROMATIC COMPOUNDS A STUDENT SHOULD BE ABLE TO: 1
... Predict whether compounds and ions are aromatic, nonaromatic or antiaromatic. First check to see if the compound has a planar, cyclic pi system. If it’s not cyclic, not planar, or has a tetrahedral atom in the ring, the ring is not aromatic. For our purposes, assume that a conjugated ring of 6 carbo ...
... Predict whether compounds and ions are aromatic, nonaromatic or antiaromatic. First check to see if the compound has a planar, cyclic pi system. If it’s not cyclic, not planar, or has a tetrahedral atom in the ring, the ring is not aromatic. For our purposes, assume that a conjugated ring of 6 carbo ...
Organic and Biochemical Compounds
... • Arrangements of carbon atoms in alkanes may vary. – The carbon atoms in any alkane with more than three carbon atoms can have more than one possible arrangement. – Carbon atom chains may have many branches, and they can even form rings. • Alkane chemical formulas usually follow a pattern. – The nu ...
... • Arrangements of carbon atoms in alkanes may vary. – The carbon atoms in any alkane with more than three carbon atoms can have more than one possible arrangement. – Carbon atom chains may have many branches, and they can even form rings. • Alkane chemical formulas usually follow a pattern. – The nu ...
Aldehydes Ketones
... Direct addition (aka 1,2 addition) occurs when a nucleophile attacks the carbon in the carbonyl directly. Conjugate addition (aka 1,4 addition) occurs when the nucleophile attacks the carbonyl indirectly by attacking the second carbon away from the carbonyl group, called the beta carbon, in an u ...
... Direct addition (aka 1,2 addition) occurs when a nucleophile attacks the carbon in the carbonyl directly. Conjugate addition (aka 1,4 addition) occurs when the nucleophile attacks the carbonyl indirectly by attacking the second carbon away from the carbonyl group, called the beta carbon, in an u ...
Organics – Naming the Molecules
... similar to naming alkenes and alkynes. This is because the location of the –OH group on the chain is ...
... similar to naming alkenes and alkynes. This is because the location of the –OH group on the chain is ...
Esters A class of organic compounds that react with water to
... A class of organic compounds that react with water to produce alcohols and organic and inorganic acids. It is mainly result of condensation of a carboxylic acid and an alcohol. ...
... A class of organic compounds that react with water to produce alcohols and organic and inorganic acids. It is mainly result of condensation of a carboxylic acid and an alcohol. ...
4.14 Halogenation of Alkanes RH + X2 → RX + HX RH + X2 → RX +
... Which of the following best describes a mechanistic feature of the free-radical bromination (Br2, light) of 2methylpropane? A) The initiation step involves cleavage of a C-H bond. B) The free-radical (CH3)3C· is produced in one propagation step and reacts with Br 2 in another. C) The reaction is cha ...
... Which of the following best describes a mechanistic feature of the free-radical bromination (Br2, light) of 2methylpropane? A) The initiation step involves cleavage of a C-H bond. B) The free-radical (CH3)3C· is produced in one propagation step and reacts with Br 2 in another. C) The reaction is cha ...
An Introduction to Functional Groups in Organic Chemistry What are
... functional group in-and-of itself; rather, it is the essential portion of two functional groups: aldehydes and ketones. ...
... functional group in-and-of itself; rather, it is the essential portion of two functional groups: aldehydes and ketones. ...
A-level Paper 2 Practice Paper 6 - A
... When the initial concentration of C is 4.55 × 10–2 mol dm–3 and the initial concentration of D is 1.70 × 10–2 mol dm–3, the initial rate of reaction is 6.64 × 10–5 mol dm–3 s–1. Calculate the value of the rate constant at this temperature and deduce its units. ...
... When the initial concentration of C is 4.55 × 10–2 mol dm–3 and the initial concentration of D is 1.70 × 10–2 mol dm–3, the initial rate of reaction is 6.64 × 10–5 mol dm–3 s–1. Calculate the value of the rate constant at this temperature and deduce its units. ...
File
... • The names of all carboxylic acids end in ‘-oic acid’ • The functional group of the carboxylic acid is -COOH (carboxyl group) • This carboxyl group is always attached to the end carbon • Carboxylic acids are polar and therefore dissolve in water • The O-H part of the carboxyl group provides hydroge ...
... • The names of all carboxylic acids end in ‘-oic acid’ • The functional group of the carboxylic acid is -COOH (carboxyl group) • This carboxyl group is always attached to the end carbon • Carboxylic acids are polar and therefore dissolve in water • The O-H part of the carboxyl group provides hydroge ...
Chapter 20. Aldehydes and Ketones
... compound (C6H5)2CHOH by adding C6H5MgBr to benzaldehyde. To insure that the chemical yield would be high, our dedicated student prepared one mole of the Grignard reagent, added two moles of benzaldehyde, and, after working up the reaction, was delighted to obtain a good yield of a crystalline produc ...
... compound (C6H5)2CHOH by adding C6H5MgBr to benzaldehyde. To insure that the chemical yield would be high, our dedicated student prepared one mole of the Grignard reagent, added two moles of benzaldehyde, and, after working up the reaction, was delighted to obtain a good yield of a crystalline produc ...
Alcohols: Structure and Physical Properties
... As we saw in the last chapter, the most important reactions of alkenes are addition reactions. Addition of a water molecule to the carbon-carbon double bond of an alkene produces an ...
... As we saw in the last chapter, the most important reactions of alkenes are addition reactions. Addition of a water molecule to the carbon-carbon double bond of an alkene produces an ...
Botany 201 - JustOnly.com
... 4. Write line angle formulas of open chain, cyclic and aromatic compounds 5. Visualize structures of alkanes, alkenes, alkynes, alkyl halides, alcohols, ethers and aromatic compounds in three dimensions and draw and construct model structures as three dimensional representations. 6. Document the rel ...
... 4. Write line angle formulas of open chain, cyclic and aromatic compounds 5. Visualize structures of alkanes, alkenes, alkynes, alkyl halides, alcohols, ethers and aromatic compounds in three dimensions and draw and construct model structures as three dimensional representations. 6. Document the rel ...
Chapter 19. Aldehydes and Ketones
... H2NNH2 and KOH converts the compound to an alkane Originally carried out at high temperatures but with dimethyl sulfoxide as solvent takes place near room temperature ...
... H2NNH2 and KOH converts the compound to an alkane Originally carried out at high temperatures but with dimethyl sulfoxide as solvent takes place near room temperature ...
General properties of urea : It is water
... Classically it was the first naturally occurring “organic” compound to be prepared from inorganic compounds traversing for the first time the great divide that appeared to classify all substances known at the time. Evaporation of an aqueous solution of ammonium cyanate to dryness was observed to giv ...
... Classically it was the first naturally occurring “organic” compound to be prepared from inorganic compounds traversing for the first time the great divide that appeared to classify all substances known at the time. Evaporation of an aqueous solution of ammonium cyanate to dryness was observed to giv ...
Wed March 3 lecture
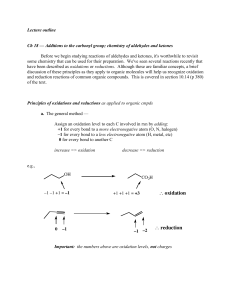
... Before we begin studying reactions of aldehydes and ketones, it's worthwhile to revisit some chemistry that can be used for their preparation. We've seen several reactions recently that have been described as oxidations or reductions. Although these are familiar concepts, a brief discussion of these ...
... Before we begin studying reactions of aldehydes and ketones, it's worthwhile to revisit some chemistry that can be used for their preparation. We've seen several reactions recently that have been described as oxidations or reductions. Although these are familiar concepts, a brief discussion of these ...
Introduction to Chemical Reactions
... Chemical reaction is a change in which one or more substances are converted into new substances The substances that react are called reactants The new substances produced are called products This relationship can be written as: ...
... Chemical reaction is a change in which one or more substances are converted into new substances The substances that react are called reactants The new substances produced are called products This relationship can be written as: ...
Excercises 6-10
... 1. The following chemical transformation of 2-‐bromo-‐1-‐phenylpropane to (E)-‐1-‐ phenylprop-‐1-‐ene is an elimination reaction. These β-‐hydrogen eliminations can proceed via three different mechanisms. Please p ...
... 1. The following chemical transformation of 2-‐bromo-‐1-‐phenylpropane to (E)-‐1-‐ phenylprop-‐1-‐ene is an elimination reaction. These β-‐hydrogen eliminations can proceed via three different mechanisms. Please p ...
Alkene

In organic chemistry, an alkene is an unsaturated hydrocarbon that contains at least one carbon–carbon double bond. Alkene, olefin, and olefine are used often interchangeably (see nomenclature section below). Acyclic alkenes, with only one double bond and no other functional groups, known as mono-enes, form a homologous series of hydrocarbons with the general formula CnH2n. Alkenes have two hydrogen atoms less than the corresponding alkane (with the same number of carbon atoms). The simplest alkene, ethylene (C2H4), which has the International Union of Pure and Applied Chemistry (IUPAC) name ethene is the organic compound produced on the largest scale industrially. Aromatic compounds are often drawn as cyclic alkenes, but their structure and properties are different and they are not considered to be alkenes.