Carboxylates/esters vs ketones/aldehydes
... BH3 becomes B(OC2H5)3 by reacting with ethanol, then, when heated with water, becomes B(OH)3. The mechanism of the NaBH4 reduction in a protic solvent such as ethanol, methanol, and water is known to be quite complex since NaBH4 reacts with the solvent, e.g., NaBH4 + C2H5OH → NaBH3(OC2H5) + H2 Becau ...
... BH3 becomes B(OC2H5)3 by reacting with ethanol, then, when heated with water, becomes B(OH)3. The mechanism of the NaBH4 reduction in a protic solvent such as ethanol, methanol, and water is known to be quite complex since NaBH4 reacts with the solvent, e.g., NaBH4 + C2H5OH → NaBH3(OC2H5) + H2 Becau ...
Ch. 09 Alcohols, Ethers, Epoxides
... and relieving the strain of the three-membered ring. • In step [2], the alkoxide is protonated with water to generate a neutral product with two functional groups on adjacent atoms. • Common nucleophiles that open the epoxide ring include ¯OH, ¯OR, ¯CN, ¯SR, and NH3. ...
... and relieving the strain of the three-membered ring. • In step [2], the alkoxide is protonated with water to generate a neutral product with two functional groups on adjacent atoms. • Common nucleophiles that open the epoxide ring include ¯OH, ¯OR, ¯CN, ¯SR, and NH3. ...
6.5. alcohols
... Air oxidises the ethanol produced to ethanoic acid (vinegar). The solution produced by fermentation will only contain around 15% ethanol. Above this concentration of ethanol the yeast will not survive. Therefore to get pure ethanol it is necessary to fractionally distil the solution made by fermenta ...
... Air oxidises the ethanol produced to ethanoic acid (vinegar). The solution produced by fermentation will only contain around 15% ethanol. Above this concentration of ethanol the yeast will not survive. Therefore to get pure ethanol it is necessary to fractionally distil the solution made by fermenta ...
Crown Ethers
... displace a halide ion and form a ring. • Treatment of a halohydrin with a base leads to an epoxide through this internal SN2 attack. ...
... displace a halide ion and form a ring. • Treatment of a halohydrin with a base leads to an epoxide through this internal SN2 attack. ...
Hydrocarbons
... once used as refrigerant gases (CFCs) Reactions We don’t have to worry about reactions of haloalkanes in this section of work ...
... once used as refrigerant gases (CFCs) Reactions We don’t have to worry about reactions of haloalkanes in this section of work ...
Chapter 3 Properties of organic compounds
... Alkanes slowly decolourise orange Br2 solution in the presence of UV light. The reaction is a substitution and the products of a monosubstitution reaction are a monobromoalkane and hydrogen bromide (an acidic gas which turns moist blue litmus paper pink). UV CH3CH2CH2CH2CH3 + Br2 $ CH3CH2CH2CH2CH2Br ...
... Alkanes slowly decolourise orange Br2 solution in the presence of UV light. The reaction is a substitution and the products of a monosubstitution reaction are a monobromoalkane and hydrogen bromide (an acidic gas which turns moist blue litmus paper pink). UV CH3CH2CH2CH2CH3 + Br2 $ CH3CH2CH2CH2CH2Br ...
Functional Group Chemistry
... electrons) while others act as electrophiles (accepting electrons). Finally, multiple bonds, as in alkenes and alkynes, are required for addition reactions. This predictable reactivity allows a chemist to attempt a variety of reactions on an organic compound and determine what functional group(s) ar ...
... electrons) while others act as electrophiles (accepting electrons). Finally, multiple bonds, as in alkenes and alkynes, are required for addition reactions. This predictable reactivity allows a chemist to attempt a variety of reactions on an organic compound and determine what functional group(s) ar ...
Alcohols Oxidation by oxygen O2 in presence of
... toluene solvent has been refluxed under oxygen atmosphere and correlated carbonyl compound was formed with high yield as the only product as showing in following. ...
... toluene solvent has been refluxed under oxygen atmosphere and correlated carbonyl compound was formed with high yield as the only product as showing in following. ...
Biology II Honors Chapter 4 Carbon and Molecular Diversity Guided
... • Living organisms consist mostly of ________________ compounds • ________________ is unparalleled in its ability to form large, complex, and diverse molecules • Proteins, DNA, carbohydrates, and other molecules that distinguish living matter are all composed of carbon compounds ...
... • Living organisms consist mostly of ________________ compounds • ________________ is unparalleled in its ability to form large, complex, and diverse molecules • Proteins, DNA, carbohydrates, and other molecules that distinguish living matter are all composed of carbon compounds ...
f3234 mod 1 revision guide rings acids and amines
... In the above reaction the methanol is in excess so the equilibrium reaction shifts to right It can be argued that the biodiesel produced from this method is classed as carbon–neutral as any carbon dioxide given off when the biofuel is burnt would have been extracted from the air by photosynthesis wh ...
... In the above reaction the methanol is in excess so the equilibrium reaction shifts to right It can be argued that the biodiesel produced from this method is classed as carbon–neutral as any carbon dioxide given off when the biofuel is burnt would have been extracted from the air by photosynthesis wh ...
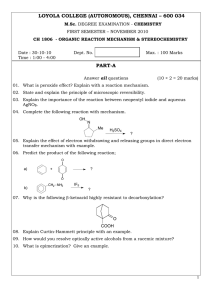
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034 PART-A
... 11. Give an example for α, β, γ and δ-elimination reaction. 12. State and explain the Hammond postulate to the bromination of n-propane. 13. How will you determine the reaction mechanism of hydrolysis of an ester using isotoping labeling method? 14. Write and explain the Steven’s rearrangement. 15. ...
... 11. Give an example for α, β, γ and δ-elimination reaction. 12. State and explain the Hammond postulate to the bromination of n-propane. 13. How will you determine the reaction mechanism of hydrolysis of an ester using isotoping labeling method? 14. Write and explain the Steven’s rearrangement. 15. ...
Name HOMEWORK PACKET - CARBON: THE ELEMENT OF LIFE
... 5. The functional group, where one or more of the hydrogen atoms are replaced by atoms from the halogen family, are called ____________. 6. The alcohols are in a functional group called the ____________ group. 7. All ____________ fuels are composed of organic compounds. 8. Polymers are made up of s ...
... 5. The functional group, where one or more of the hydrogen atoms are replaced by atoms from the halogen family, are called ____________. 6. The alcohols are in a functional group called the ____________ group. 7. All ____________ fuels are composed of organic compounds. 8. Polymers are made up of s ...
File - Garbally Chemistry
... 1. Ethane is produced in small amounts. Its occurrence can only be explained by CH3+ CH3 CH3CH3 If the reaction is left run with excess chlorine and uv light di- tri- and tetra-chloro methane are produced as are minute amounts of a range of chloroethanes. 2. The presence of tetramethyl-lead greatly ...
... 1. Ethane is produced in small amounts. Its occurrence can only be explained by CH3+ CH3 CH3CH3 If the reaction is left run with excess chlorine and uv light di- tri- and tetra-chloro methane are produced as are minute amounts of a range of chloroethanes. 2. The presence of tetramethyl-lead greatly ...
Acyl Anions Derived from Enol Ethers
... The normal disconnection pattern of a carboxylic acid with a Grignard reagent and carbon dioxide as SEs (path a) and a disconnection leading to a carboxyl synthon with an "unnatural" negative charge (path b). Cyanide ion can act as an SE of a negatively charged carboxyl synthon. Its reaction with R ...
... The normal disconnection pattern of a carboxylic acid with a Grignard reagent and carbon dioxide as SEs (path a) and a disconnection leading to a carboxyl synthon with an "unnatural" negative charge (path b). Cyanide ion can act as an SE of a negatively charged carboxyl synthon. Its reaction with R ...
8.1 Alcohols, Phenols, and Ethers
... ketones, the “–e” is dropped from the alkane containing the carbonyl group and replaced with the suffix “-one”. The location of the carbonyl group must be specified in ketones containing five or more carbons. The chain is numbered in a manner which places the lowest number on the carbon containing t ...
... ketones, the “–e” is dropped from the alkane containing the carbonyl group and replaced with the suffix “-one”. The location of the carbonyl group must be specified in ketones containing five or more carbons. The chain is numbered in a manner which places the lowest number on the carbon containing t ...
Carbonyl Compounds
... Explain the order of reactivities of aldehydes and ketones towards nucleophilic addition reaction:in terms of electronic effect and steric effect of alkyl group: . HCHO > RCHO >RCR’O >CHO >CRO > CO ( REFERS TO BENZENE) ...
... Explain the order of reactivities of aldehydes and ketones towards nucleophilic addition reaction:in terms of electronic effect and steric effect of alkyl group: . HCHO > RCHO >RCR’O >CHO >CRO > CO ( REFERS TO BENZENE) ...
1072. A General Synthesis of Ethers.
... solution, hydrogenation of a ketal gives the same results as that of the carbonyl compound. Enolethers such as 1-methoxycyclopentene also give a mixture of alkane and saturated ether, but in a ratio different from that found for direct hydrogenation of cyclopentanone in acid methanol. This could be ...
... solution, hydrogenation of a ketal gives the same results as that of the carbonyl compound. Enolethers such as 1-methoxycyclopentene also give a mixture of alkane and saturated ether, but in a ratio different from that found for direct hydrogenation of cyclopentanone in acid methanol. This could be ...
Protecting Groups Introduction to Carbonyl
... Protecting Groups Solving this problem requires a three-step strategy: [1] Convert the OH group into another functional group that does not interfere with the desired reaction. This new blocking group is called a protecting group, and the reaction that creates it is called “protection.” [2] Carry ou ...
... Protecting Groups Solving this problem requires a three-step strategy: [1] Convert the OH group into another functional group that does not interfere with the desired reaction. This new blocking group is called a protecting group, and the reaction that creates it is called “protection.” [2] Carry ou ...
File
... 2) Alcohols which have 3 OH goups are called triols CH2OHCHOHCH2OH = 1,2, 3-propanetriol or glycerol or glycerin ...
... 2) Alcohols which have 3 OH goups are called triols CH2OHCHOHCH2OH = 1,2, 3-propanetriol or glycerol or glycerin ...
CH 18 blackboard
... In alkane substitution reactions, one or more hydrogen atoms on an alkane are replaced by one or more other types of atoms. The most common substitution reaction is halogen substitution. Methane reacts with chlorine gas to form chloromethane. ...
... In alkane substitution reactions, one or more hydrogen atoms on an alkane are replaced by one or more other types of atoms. The most common substitution reaction is halogen substitution. Methane reacts with chlorine gas to form chloromethane. ...
Alkene

In organic chemistry, an alkene is an unsaturated hydrocarbon that contains at least one carbon–carbon double bond. Alkene, olefin, and olefine are used often interchangeably (see nomenclature section below). Acyclic alkenes, with only one double bond and no other functional groups, known as mono-enes, form a homologous series of hydrocarbons with the general formula CnH2n. Alkenes have two hydrogen atoms less than the corresponding alkane (with the same number of carbon atoms). The simplest alkene, ethylene (C2H4), which has the International Union of Pure and Applied Chemistry (IUPAC) name ethene is the organic compound produced on the largest scale industrially. Aromatic compounds are often drawn as cyclic alkenes, but their structure and properties are different and they are not considered to be alkenes.