Organic Chemistry - GZ @ Science Class Online
... Alkenes undergo addition reactions - this means they can undergo addition of a halogen atom (chlorine, bromine, iodine) across the double bond to form a haloalkane. The common test for an unsaturated hydrocarbon (alkene) to distinguish it from a saturated hydrocarbon (alkane) is therefore the rapid ...
... Alkenes undergo addition reactions - this means they can undergo addition of a halogen atom (chlorine, bromine, iodine) across the double bond to form a haloalkane. The common test for an unsaturated hydrocarbon (alkene) to distinguish it from a saturated hydrocarbon (alkane) is therefore the rapid ...
functional groups NOTES kelly
... • Living matter-mainly of carbon (C), oxygen (O), hydrogen (H), and nitrogen (N) with smaller amounts of sulfur (S) and phosphorus (P). SINGLE BOND shares pair of electrons ...
... • Living matter-mainly of carbon (C), oxygen (O), hydrogen (H), and nitrogen (N) with smaller amounts of sulfur (S) and phosphorus (P). SINGLE BOND shares pair of electrons ...
Chapter 1_part 2
... this bond's weakness is explained by significantly less overlap between the component porbitals due to their parallel orientation. This is contrasted by sigma bond which form bonding orbitals directly between the nucleus of the bonding atoms, resulting in greater overlap and a strong sigma bond. ...
... this bond's weakness is explained by significantly less overlap between the component porbitals due to their parallel orientation. This is contrasted by sigma bond which form bonding orbitals directly between the nucleus of the bonding atoms, resulting in greater overlap and a strong sigma bond. ...
organic chem notes
... impossible to rotate the bond without breaking it. Geometric isomers are isomers that differ from each other based on the position of the attached groups relative to the double bond. Two substituents that are on the same side of the double bond is called cis, meaning "same", two substituents that ar ...
... impossible to rotate the bond without breaking it. Geometric isomers are isomers that differ from each other based on the position of the attached groups relative to the double bond. Two substituents that are on the same side of the double bond is called cis, meaning "same", two substituents that ar ...
Naming organic compounds
... For more than one branch, the branches are identified in alphabetical order, ignoring any 'di', 'tri', etc, prefixes. Each branch needs to be numbered individually, even if they are attached to the same carbon atom. The rule is a comma between numbers, and a dash between numbers and letters. Naming ...
... For more than one branch, the branches are identified in alphabetical order, ignoring any 'di', 'tri', etc, prefixes. Each branch needs to be numbered individually, even if they are attached to the same carbon atom. The rule is a comma between numbers, and a dash between numbers and letters. Naming ...
Structure and Bonding in Organic Compounds
... While organic chemistry is a vast subject, we will concern ourselves with one fundamental concepts that will form a solid foundation for those students who will be continuing with Chem 104 or Chem 150: an understanding of the structure and bonding in organic compounds. This experiment is designed to ...
... While organic chemistry is a vast subject, we will concern ourselves with one fundamental concepts that will form a solid foundation for those students who will be continuing with Chem 104 or Chem 150: an understanding of the structure and bonding in organic compounds. This experiment is designed to ...
Organic Chemistry Control Test
... 4.4 state one possible reason for the trend mentioned in 6.3 4.5 The boiling points of alcohols are notably higher than that of alkanes. Give a reason for this. ...
... 4.4 state one possible reason for the trend mentioned in 6.3 4.5 The boiling points of alcohols are notably higher than that of alkanes. Give a reason for this. ...
Chapter 26 Functional Groups and Organic Reactions
... it is said to be unsaturated (meaning that it would be possible to fit in more hydrogens if the double bonds were changed to single). Chains with one double bond take the suffix ene. Example: ethene CH2=CH2 Chains with one triple bond take the suffix – yne. Example: Ethyne CHΞCH ...
... it is said to be unsaturated (meaning that it would be possible to fit in more hydrogens if the double bonds were changed to single). Chains with one double bond take the suffix ene. Example: ethene CH2=CH2 Chains with one triple bond take the suffix – yne. Example: Ethyne CHΞCH ...
Chemistry
... Exothermic and endothermic processes; the Joule as a unit of energy. Thermochemical equations and the ∆H notation. Energy changes accompanying neutralisation, solution, combustion and atomisation reactions. Experimental determination of energy changes not required. Calorific value of fuels and food; ...
... Exothermic and endothermic processes; the Joule as a unit of energy. Thermochemical equations and the ∆H notation. Energy changes accompanying neutralisation, solution, combustion and atomisation reactions. Experimental determination of energy changes not required. Calorific value of fuels and food; ...
Chemistry 201 - Department of Chemistry | Oregon State University
... Amino acids have the ability to link together and form proteins. Amino acids have carboxylic acid groups Amino acids have a base and an acid in the same molecule There are about 20 amino acids each of which has a different sidechain that yield different properties. All of the amino acids are nonpola ...
... Amino acids have the ability to link together and form proteins. Amino acids have carboxylic acid groups Amino acids have a base and an acid in the same molecule There are about 20 amino acids each of which has a different sidechain that yield different properties. All of the amino acids are nonpola ...
Chemistry
... Exothermic and endothermic processes; the Joule as a unit of energy. Thermochemical equations and the ∆H notation. Energy changes accompanying neutralisation, solution, combustion and atomisation reactions. Experimental determination of energy changes not required. Calorific value of fuels and food; ...
... Exothermic and endothermic processes; the Joule as a unit of energy. Thermochemical equations and the ∆H notation. Energy changes accompanying neutralisation, solution, combustion and atomisation reactions. Experimental determination of energy changes not required. Calorific value of fuels and food; ...
alcohols - GCG-42
... Lucas reagent : equimolar mixture of c.HCl and anhyd. ZnCl2 Appearance of cloudiness in the rxn mixture indicates the conversion of alcohol into alkyl halide. Observation30 alcohol:- reacts immediately & cloudiness appears ...
... Lucas reagent : equimolar mixture of c.HCl and anhyd. ZnCl2 Appearance of cloudiness in the rxn mixture indicates the conversion of alcohol into alkyl halide. Observation30 alcohol:- reacts immediately & cloudiness appears ...
Exam 3 Key
... 1. The condition of an atom that has at least one of its electrons in orbitals that do not represent the lowest possible potential energy is called a(n) excited state. 2. A(n) antibonding molecular orbital is formed from out-of-phase interaction of two atomic orbitals. This leads to a decrease in ne ...
... 1. The condition of an atom that has at least one of its electrons in orbitals that do not represent the lowest possible potential energy is called a(n) excited state. 2. A(n) antibonding molecular orbital is formed from out-of-phase interaction of two atomic orbitals. This leads to a decrease in ne ...
CHEMISTRY IM 06 SYLLABUS 1
... Exothermic and endothermic processes; the Joule as a unit of energy. Thermochemical equations and the H notation. Energy changes accompanying neutralisation, solution, combustion and atomisation reactions. Experimental determination of energy changes not required. Calorific value of fuels and food; ...
... Exothermic and endothermic processes; the Joule as a unit of energy. Thermochemical equations and the H notation. Energy changes accompanying neutralisation, solution, combustion and atomisation reactions. Experimental determination of energy changes not required. Calorific value of fuels and food; ...
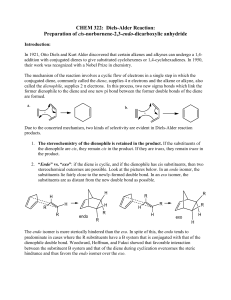
Diels-Alder Reaction:
... Introduction: In 1921, Otto Diels and Kurt Alder discovered that certain alkenes and alkynes can undergo a 1,4addition with conjugated dienes to give substituted cyclohexenes or 1,4-cyclohexadienes. In 1950, their work was recognized with a Nobel Prize in chemistry. The mechanism of the reaction inv ...
... Introduction: In 1921, Otto Diels and Kurt Alder discovered that certain alkenes and alkynes can undergo a 1,4addition with conjugated dienes to give substituted cyclohexenes or 1,4-cyclohexadienes. In 1950, their work was recognized with a Nobel Prize in chemistry. The mechanism of the reaction inv ...
Chapter23
... Ethanol is the intoxicating substance in alcoholic beverages. It is a depressant that can be fatal if taken in large doses at once. Denatured alcohol is ethanol with an added substance (CH4OH or wood alcohol) to make it toxic (poisonous). 4. Addition Reactions - Addition reactions of alkenes are an ...
... Ethanol is the intoxicating substance in alcoholic beverages. It is a depressant that can be fatal if taken in large doses at once. Denatured alcohol is ethanol with an added substance (CH4OH or wood alcohol) to make it toxic (poisonous). 4. Addition Reactions - Addition reactions of alkenes are an ...
CHEMISTRY 105
... red chains are both four C’s long. In the third structure, the red chain is 5 C’s long. The longest chain isn’t always left to right. It just happened that way here. But it is necessary to find the longest carbon chain first. The name of this parent chain will be pentane (3methylpentane, to give the ...
... red chains are both four C’s long. In the third structure, the red chain is 5 C’s long. The longest chain isn’t always left to right. It just happened that way here. But it is necessary to find the longest carbon chain first. The name of this parent chain will be pentane (3methylpentane, to give the ...
Practice Exam 2 - Department of Chemistry and Biochemistry
... In a molecule with covalent bonding, A) atoms are held together by sharing electrons. B) oppositely charged ions are held together by strong electrical attractions. C) atoms of different metals form bonds. D) atoms of noble gases are held together by attractions between oppositely charged ions. E) a ...
... In a molecule with covalent bonding, A) atoms are held together by sharing electrons. B) oppositely charged ions are held together by strong electrical attractions. C) atoms of different metals form bonds. D) atoms of noble gases are held together by attractions between oppositely charged ions. E) a ...
Halogenoalkanes
... hydroxide ion, OH–, is a nucleophile and is attracted to the slight positive charge on the C of the polar C-Br bond. Draw the mechanism of this nucleophilic substitution reaction below: ...
... hydroxide ion, OH–, is a nucleophile and is attracted to the slight positive charge on the C of the polar C-Br bond. Draw the mechanism of this nucleophilic substitution reaction below: ...
Biochemistry Worksheet
... covalent bond? triple covalent bond? quadruple covalent bond? 8. Explain what is meant by a functional group, & tell what effect they have on the molecules they are attached to. 9. Write the formula for these functional groups (use your textbook) --hydroxyl, carboxyl, phosphate group, and amino grou ...
... covalent bond? triple covalent bond? quadruple covalent bond? 8. Explain what is meant by a functional group, & tell what effect they have on the molecules they are attached to. 9. Write the formula for these functional groups (use your textbook) --hydroxyl, carboxyl, phosphate group, and amino grou ...
Chapter One: Molecular Structure
... reaction between ethers and epoxides with nucleophiles under acidic and basic conditions. Predict the stereochemistry and optical activity of a product from an understanding of its mechanism of formation. Propose a reaction or sequence of reactions to produce a target ether or epoxide in high yi ...
... reaction between ethers and epoxides with nucleophiles under acidic and basic conditions. Predict the stereochemistry and optical activity of a product from an understanding of its mechanism of formation. Propose a reaction or sequence of reactions to produce a target ether or epoxide in high yi ...
Alkene

In organic chemistry, an alkene is an unsaturated hydrocarbon that contains at least one carbon–carbon double bond. Alkene, olefin, and olefine are used often interchangeably (see nomenclature section below). Acyclic alkenes, with only one double bond and no other functional groups, known as mono-enes, form a homologous series of hydrocarbons with the general formula CnH2n. Alkenes have two hydrogen atoms less than the corresponding alkane (with the same number of carbon atoms). The simplest alkene, ethylene (C2H4), which has the International Union of Pure and Applied Chemistry (IUPAC) name ethene is the organic compound produced on the largest scale industrially. Aromatic compounds are often drawn as cyclic alkenes, but their structure and properties are different and they are not considered to be alkenes.