CLASS X carbon and its compound
... 1. Covalent bond or Molecular bond or Homopolar bond : A chemical bond formed between two non-metallic elements by the mutual sharing of one or more electron pairs is called covalent bond. 2. Covalency : The number of electron pairs which an atom of an element mutually shares with another atom or at ...
... 1. Covalent bond or Molecular bond or Homopolar bond : A chemical bond formed between two non-metallic elements by the mutual sharing of one or more electron pairs is called covalent bond. 2. Covalency : The number of electron pairs which an atom of an element mutually shares with another atom or at ...
ketones - Fisanti Site
... primary alcohols. • Organometallics with carbon dioxide yield carboxylic acids. ...
... primary alcohols. • Organometallics with carbon dioxide yield carboxylic acids. ...
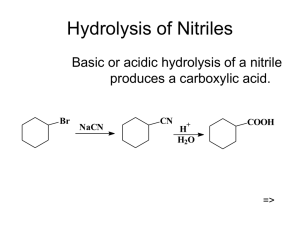
Hydrolysis of Nitriles
... Chloride is a good leaving group, so undergoes acyl substitution easily. To synthesize acid chlorides use thionyl chloride or oxalyl chloride with the acid. O ...
... Chloride is a good leaving group, so undergoes acyl substitution easily. To synthesize acid chlorides use thionyl chloride or oxalyl chloride with the acid. O ...
Aromatic amines The
... A Nitrogen, containing a lone pair is the key atom Resembles Ammonia where one or more H’s Are replaced by alkyl groups The lone pair participates in the reactivity Of amines Amines are a core part of ‘amino acids’ ...
... A Nitrogen, containing a lone pair is the key atom Resembles Ammonia where one or more H’s Are replaced by alkyl groups The lone pair participates in the reactivity Of amines Amines are a core part of ‘amino acids’ ...
calculations-questions-part
... *(v) For Method 2, describe the practical steps that you would take to obtain pure dry crystals of hydrated nickel(II) nitrate, Ni(NO3)2.6H2O, from a mixture of nickel(II) nitrate solution and unreacted solid ...
... *(v) For Method 2, describe the practical steps that you would take to obtain pure dry crystals of hydrated nickel(II) nitrate, Ni(NO3)2.6H2O, from a mixture of nickel(II) nitrate solution and unreacted solid ...
Overview of the Reactions of Carbonyl Compounds
... Oxidation of Aldehydes and Ketones • Aldehydes are readily oxidized to carboxylic acid but ketones are unreactive towards oxidation except under the most vigorous conditions. This difference in reactivity towards oxidation lies in the structural difference between the two types of carbonyl compound ...
... Oxidation of Aldehydes and Ketones • Aldehydes are readily oxidized to carboxylic acid but ketones are unreactive towards oxidation except under the most vigorous conditions. This difference in reactivity towards oxidation lies in the structural difference between the two types of carbonyl compound ...
6.1.3 revision guide carboxylic acids and esters
... withdraw electron density from the COO- ion, making it less negative and more stable. This make the acid more strong. ...
... withdraw electron density from the COO- ion, making it less negative and more stable. This make the acid more strong. ...
Oxidation of Alcohols
... 15.7: Conversion of Alcohols to Ethers - Symmetrical ethers can be prepared by treating the corresponding alcohol with a strong acid. H3CH2C-OH + HO-CH2CH3 ...
... 15.7: Conversion of Alcohols to Ethers - Symmetrical ethers can be prepared by treating the corresponding alcohol with a strong acid. H3CH2C-OH + HO-CH2CH3 ...
3.5 The Alcohols
... The more electronegative oxygen produces a polar bond This makes the alcohols more reactive ...
... The more electronegative oxygen produces a polar bond This makes the alcohols more reactive ...
AS 2, Module 2
... (a) A sample of the wine was subjected to infra-red spectroscopy and the results compared to the spectra for ethanol, ethanal and ethanoic acid. The infra-red spectra for ethanol, ethanal and ethanoic acid are shown below (not necessarily in that order). ...
... (a) A sample of the wine was subjected to infra-red spectroscopy and the results compared to the spectra for ethanol, ethanal and ethanoic acid. The infra-red spectra for ethanol, ethanal and ethanoic acid are shown below (not necessarily in that order). ...
Hydrocarbon Derivatives - AHS-SCH4U
... 1. Locate the longest carbon chain (must contain the carbon attached to the –OH group.) Name the parent alkane 2. The suffix is –ol; indicate the position of the –OH group in front of the suffix 3. More than one –OH group; use di, tri, tetra and keep entire root name 4. Number the chain so that the ...
... 1. Locate the longest carbon chain (must contain the carbon attached to the –OH group.) Name the parent alkane 2. The suffix is –ol; indicate the position of the –OH group in front of the suffix 3. More than one –OH group; use di, tri, tetra and keep entire root name 4. Number the chain so that the ...
Hydrogen bonding
... A tertiary alcohol reacts if it is shaken with concentrated hydrochloric acid at room temperature . This reaction occurs by SN1 mechanism, so the reaction rate is almost the same with HCl, HBr or HI, since the addition of the halide nucleophile occurs in the second fast step. ...
... A tertiary alcohol reacts if it is shaken with concentrated hydrochloric acid at room temperature . This reaction occurs by SN1 mechanism, so the reaction rate is almost the same with HCl, HBr or HI, since the addition of the halide nucleophile occurs in the second fast step. ...
Abstract OXIDATIVE TRANSFORMATIONS AND CYCLIZATIONS
... halogen based reagents. Chapter I deals with the importance of halogens in organic reactions, emphasizing more on their use for oxidative transformations. Further this chapter focuses on the properties and applications of iodine based reagents for various organic transformations. Emphasis on catalyt ...
... halogen based reagents. Chapter I deals with the importance of halogens in organic reactions, emphasizing more on their use for oxidative transformations. Further this chapter focuses on the properties and applications of iodine based reagents for various organic transformations. Emphasis on catalyt ...
OR Practice Problem - HCC Southeast Commons
... Alcohols and phenols have high boiling points because they form hydrogen bonds (like H2O) in solution ...
... Alcohols and phenols have high boiling points because they form hydrogen bonds (like H2O) in solution ...
Gas Chromatography: Analyzing Alkene Isomers David L. Flanigan
... using a dilute solution of sulfuric acid. The reaction resulted in a mixture of alkenes that was purified by distillation and analyzed using gas chromatography. Theoretical Background: Alcohols can be dehydrated using catalytic acid conditions to give alkenes. H+ ...
... using a dilute solution of sulfuric acid. The reaction resulted in a mixture of alkenes that was purified by distillation and analyzed using gas chromatography. Theoretical Background: Alcohols can be dehydrated using catalytic acid conditions to give alkenes. H+ ...
The Aldol Condensation Preparation of 4
... Wurtz's studies of aldehydes … The aldol condensation proved to be a general reaction of aldehydes with some ketones, provided hydrogen is present on the alpha carbon atom. In Kiel, Ludwig Claisen extended its use in several directions; he considered aldehydes with aldehydes, ketones, and esters und ...
... Wurtz's studies of aldehydes … The aldol condensation proved to be a general reaction of aldehydes with some ketones, provided hydrogen is present on the alpha carbon atom. In Kiel, Ludwig Claisen extended its use in several directions; he considered aldehydes with aldehydes, ketones, and esters und ...
Chapter 16 – Amines and Amides
... groups. The alkaloids are a class of these materials. If you see a drug end in “-ine” there is a good chance of it containing an amino group. Examples include: codeine, morphine, and quinine (all have pictures in your book on p. 486). Amines generally have an odor that is ammonia-like to fishy and m ...
... groups. The alkaloids are a class of these materials. If you see a drug end in “-ine” there is a good chance of it containing an amino group. Examples include: codeine, morphine, and quinine (all have pictures in your book on p. 486). Amines generally have an odor that is ammonia-like to fishy and m ...
Document
... • There will be two possible Wittig routes to an alkene. • Analyze the structure retrosynthetically, i.e., work the synthesis out backworks • Disconnect (break the bond of the target that can be formed by a known reaction) the doubly bonded carbons. One becomes the aldehyde or ketone, the other the ...
... • There will be two possible Wittig routes to an alkene. • Analyze the structure retrosynthetically, i.e., work the synthesis out backworks • Disconnect (break the bond of the target that can be formed by a known reaction) the doubly bonded carbons. One becomes the aldehyde or ketone, the other the ...
chm121 tutorial kit - Covenant University
... (a) the two double bonds are joined by a single bond (b) a single carbon atom is common to two double bonds (c) the two double bonds are separated from each other by one or more sp3-hybridized C-atoms (d) two single carbon atoms are common to two double bonds ...
... (a) the two double bonds are joined by a single bond (b) a single carbon atom is common to two double bonds (c) the two double bonds are separated from each other by one or more sp3-hybridized C-atoms (d) two single carbon atoms are common to two double bonds ...
Ch. 14 Alcohols, Ethers, & Thiols
... • The common naming system is used for simple ethers: – List the alkyl groups bonded to the oxygen in alphabetical order, followed by the work “ether”. H 3C ...
... • The common naming system is used for simple ethers: – List the alkyl groups bonded to the oxygen in alphabetical order, followed by the work “ether”. H 3C ...
Exp`t 88 - Chemistry Courses
... Ester syntheses are one of the more enjoyable experiments in organic chemistry, as one can't help but notice the special odors associated with esters - some fruity, some more like nail polish. In contrast, the acids which are used in their syntheses usually have a rotten odor. The reverse reaction, ...
... Ester syntheses are one of the more enjoyable experiments in organic chemistry, as one can't help but notice the special odors associated with esters - some fruity, some more like nail polish. In contrast, the acids which are used in their syntheses usually have a rotten odor. The reverse reaction, ...
Chap20 Grignard reagents
... 1) Grignard reagents are nucleophiles that add to the specific types of electrophiles shown in this handout (ie. that are not generally used for SN 2 reactions on alkyl halides). 2) Alkyl Na, Li, and K reagents (i.e. H 3CC CNa ) react very similarly to Grignard reagents. 3) An R – addition to a carb ...
... 1) Grignard reagents are nucleophiles that add to the specific types of electrophiles shown in this handout (ie. that are not generally used for SN 2 reactions on alkyl halides). 2) Alkyl Na, Li, and K reagents (i.e. H 3CC CNa ) react very similarly to Grignard reagents. 3) An R – addition to a carb ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.