How to Name Chemical Compounds
... Inorganic acids – these compounds contain hydrogen available for donation in a chemical reaction; their formulas generally start with H (hydrogen) and are followed by a nonmetal or polyatomic anion Hydrated compounds – these compounds appear as a salt or ionic compound with water molecules attached; ...
... Inorganic acids – these compounds contain hydrogen available for donation in a chemical reaction; their formulas generally start with H (hydrogen) and are followed by a nonmetal or polyatomic anion Hydrated compounds – these compounds appear as a salt or ionic compound with water molecules attached; ...
Structure and Synthesis of Alkenes
... When similar groups (not H’s) are bound to the same side of the double bond the alkene is said to be cis. When similar groups are bound to opposite sides of the double bond it is said to be trans. ...
... When similar groups (not H’s) are bound to the same side of the double bond the alkene is said to be cis. When similar groups are bound to opposite sides of the double bond it is said to be trans. ...
Survival Organic Chemistry Part I: Molecular Models
... 1. Write the general rule for determining whether a chemical formula represents an ionic or a covalent compound. For example, which of the following formulas describe ionic and/or covalent compounds? NaCl, CO2, CaCl2, HCl, CH3Br, NH4NO3, Ba(NO3)2 2. Draw a Lewis electron-dot structure for each of th ...
... 1. Write the general rule for determining whether a chemical formula represents an ionic or a covalent compound. For example, which of the following formulas describe ionic and/or covalent compounds? NaCl, CO2, CaCl2, HCl, CH3Br, NH4NO3, Ba(NO3)2 2. Draw a Lewis electron-dot structure for each of th ...
Investigating Esters
... not be possible to determine the boiling point by the distillation method and a semi micro method should be employed. The suggested method in the starter experiment is a generic one and may need to be slightly adjusted for each ester. Students should be encouraged to find details from the literature ...
... not be possible to determine the boiling point by the distillation method and a semi micro method should be employed. The suggested method in the starter experiment is a generic one and may need to be slightly adjusted for each ester. Students should be encouraged to find details from the literature ...
Investigating Esters
... not be possible to determine the boiling point by the distillation method and a semi micro method should be employed. The suggested method in the starter experiment is a generic one and may need to be slightly adjusted for each ester. Students should be encouraged to find details from the literature ...
... not be possible to determine the boiling point by the distillation method and a semi micro method should be employed. The suggested method in the starter experiment is a generic one and may need to be slightly adjusted for each ester. Students should be encouraged to find details from the literature ...
cellulose
... THANE-l:2-diol and propane-l:3-diol obtain ring compounds, the condensation reaet with 1: 1:3:3-tetramethyl-l:3-direaction must be carried out under high diluchlorodisiloxane forming the correspondtion; otherwise poly-condensation reactions ing rings. However t no ring compounds predominate. could b ...
... THANE-l:2-diol and propane-l:3-diol obtain ring compounds, the condensation reaet with 1: 1:3:3-tetramethyl-l:3-direaction must be carried out under high diluchlorodisiloxane forming the correspondtion; otherwise poly-condensation reactions ing rings. However t no ring compounds predominate. could b ...
Survival Organic Laboratory
... single, double and triple bonds, bond lengths and angles, resonance, and bond dissociation energies. Your textbook will play an important role as a reference tool in this laboratory. Chapters and sections which will be important to refer to include; Chapter 15, sections 15.1 - 15.4 Chapter 9, sectio ...
... single, double and triple bonds, bond lengths and angles, resonance, and bond dissociation energies. Your textbook will play an important role as a reference tool in this laboratory. Chapters and sections which will be important to refer to include; Chapter 15, sections 15.1 - 15.4 Chapter 9, sectio ...
Notes: Alcohols, Ethers, Phenols and Thiols
... An ether is a hydrocarbon with an oxygen sandwiched between the carbons. They are very useful in organic chemistry. They tend to by very good solvents, much like water, and have relatively low reactivity. They are very resistant to oxidizing agents like potassium dichromate and potassium permanganat ...
... An ether is a hydrocarbon with an oxygen sandwiched between the carbons. They are very useful in organic chemistry. They tend to by very good solvents, much like water, and have relatively low reactivity. They are very resistant to oxidizing agents like potassium dichromate and potassium permanganat ...
Chapter 12: IR Spectroscopy and Mass Spectrometry
... The most important factor to remember about IR spectra is that it is a tool to help determine key functional groups. As such, you should never look to the right of 1600cm-1 (fingerprint region) of an IR spectrum. ...
... The most important factor to remember about IR spectra is that it is a tool to help determine key functional groups. As such, you should never look to the right of 1600cm-1 (fingerprint region) of an IR spectrum. ...
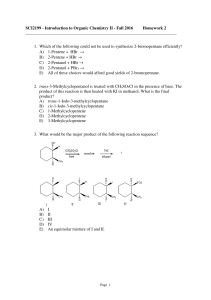
SCI2199 - Introduction to Organic Chemistry II
... 7. Which of the following could be used to synthesize 2-bromobutane? A) CH3CH2CH=CH2 + Br2(aq) → B) CH3CH2CHOHCH3 + HBr → C) CH3CH2C≡CH + HBr → D) CH3CH2C≡CH + Br2 → E) More than one of these choices. 8. Which of the alcohols listed below would you expect to react most rapidly with ...
... 7. Which of the following could be used to synthesize 2-bromobutane? A) CH3CH2CH=CH2 + Br2(aq) → B) CH3CH2CHOHCH3 + HBr → C) CH3CH2C≡CH + HBr → D) CH3CH2C≡CH + Br2 → E) More than one of these choices. 8. Which of the alcohols listed below would you expect to react most rapidly with ...
Topic 16 Test - A
... How many structural isomers, which are aldehydes, have the molecular formula C5H10O? A ...
... How many structural isomers, which are aldehydes, have the molecular formula C5H10O? A ...
Alcohols, phenols, thiols and ethers notes
... • A number giving the position of each of the hydroxyl groups is needed in these cases. ...
... • A number giving the position of each of the hydroxyl groups is needed in these cases. ...
Carboxylic Acids - MCAT Cooperative
... Electron donating groups ( next to ring) activate the ring and are ortho-para directors Electron withdrawing groups (+ or δ+ next to ring)deactivate the ring and are meta directors Halogens are electron withdrawing BUT are ...
... Electron donating groups ( next to ring) activate the ring and are ortho-para directors Electron withdrawing groups (+ or δ+ next to ring)deactivate the ring and are meta directors Halogens are electron withdrawing BUT are ...
Organic Compounds
... What are organic compounds? Organic compounds are any compounds that contain carbon (with a few exceptions). All other compounds are referred to as inorganic compounds. In almost all organic compounds, carbon atoms are bonded to hydrogen atoms or other elements that are near carbon in the periodic t ...
... What are organic compounds? Organic compounds are any compounds that contain carbon (with a few exceptions). All other compounds are referred to as inorganic compounds. In almost all organic compounds, carbon atoms are bonded to hydrogen atoms or other elements that are near carbon in the periodic t ...
Chapter 9 Compounds of carbon
... the electronegative nature of the oxygen atom. These bonds are stronger than the bonds between chloroalkane molecules and the bonds between butane molecules. Q20. Explain why alkanols such as methanol and ethanol are soluble in water in all proportions whereas the alkanols higher in the homologous s ...
... the electronegative nature of the oxygen atom. These bonds are stronger than the bonds between chloroalkane molecules and the bonds between butane molecules. Q20. Explain why alkanols such as methanol and ethanol are soluble in water in all proportions whereas the alkanols higher in the homologous s ...
US06CICV02 Unit -3 Dr. N. K. Patel Natubhai V. Patel College of
... contain unreacted methane and hydrogen chloride. These last mentioned materials are separated from the chloromethanes by scrubbing the reacted gases with a mixture of liquid chloromethanes usually a refrigerated mixture of chloroform and carbon tetrachloride. Methane and hydrogen chloride are not ab ...
... contain unreacted methane and hydrogen chloride. These last mentioned materials are separated from the chloromethanes by scrubbing the reacted gases with a mixture of liquid chloromethanes usually a refrigerated mixture of chloroform and carbon tetrachloride. Methane and hydrogen chloride are not ab ...
Lecture 7a
... acid a poor electrophile The situation changes in the protonated form of the carboxylic acid in which the carbonyl carbon bears a larger positive charge (~0.2 units in the case of acetic acid), which makes it a better electrophile ...
... acid a poor electrophile The situation changes in the protonated form of the carboxylic acid in which the carbonyl carbon bears a larger positive charge (~0.2 units in the case of acetic acid), which makes it a better electrophile ...
Alcohols phenols ethers
... (R–O/Ar–O) yields another class of compounds known as ‘ethers’, for example, CH3OCH3 (dimethyl ether). ethers as compounds formed by substituting the hydrogen atom of hydroxyl group of an alcohol or phenol by an alkyl or aryl group. Alcohols are organic compounds that have one or more hydroxy (-OH) ...
... (R–O/Ar–O) yields another class of compounds known as ‘ethers’, for example, CH3OCH3 (dimethyl ether). ethers as compounds formed by substituting the hydrogen atom of hydroxyl group of an alcohol or phenol by an alkyl or aryl group. Alcohols are organic compounds that have one or more hydroxy (-OH) ...
Lab 2 - Academic Computer Center
... with a positive reagent (electrophile). A EWG “pulls” electron density toward itself from the rest of the molecule, creating a positive center that facilitates a reaction with a negative reagent (nucleophile). Electron-Donating Groups ...
... with a positive reagent (electrophile). A EWG “pulls” electron density toward itself from the rest of the molecule, creating a positive center that facilitates a reaction with a negative reagent (nucleophile). Electron-Donating Groups ...
Alkenes
... • Alkenes are hydrocarbon with carbon-carbon double bonds. • Alkenes are also called olefins, meaning “oilforming gas”. • The functional group of alkenes is the carboncarbon double bond, which is reactive. ...
... • Alkenes are hydrocarbon with carbon-carbon double bonds. • Alkenes are also called olefins, meaning “oilforming gas”. • The functional group of alkenes is the carboncarbon double bond, which is reactive. ...
Unit 2 - Carbon Compounds
... o The alkanes are a subset of the set of hydrocarbons. o The general formula for the alkanes is CnH2n+2. o An alkane can be identified from the ‘-ane’ ending. o Straight-chain alkanes can be named from molecular formulae, shortened and full structural formulae (only C1 to C8). ...
... o The alkanes are a subset of the set of hydrocarbons. o The general formula for the alkanes is CnH2n+2. o An alkane can be identified from the ‘-ane’ ending. o Straight-chain alkanes can be named from molecular formulae, shortened and full structural formulae (only C1 to C8). ...
info
... a. Of vicinal diols – use periodic acid (HIO4). Produces aldehydes and ketones. b. Of alkenes – ozonolysis. This is a syn addition of ozone to the alkene, giving a molozonide. It then rearranges to form an ozonide (two O’s on one side, one O on the other, in a five‐membered ring). i. Oxidative ...
... a. Of vicinal diols – use periodic acid (HIO4). Produces aldehydes and ketones. b. Of alkenes – ozonolysis. This is a syn addition of ozone to the alkene, giving a molozonide. It then rearranges to form an ozonide (two O’s on one side, one O on the other, in a five‐membered ring). i. Oxidative ...
Chapter 10
... • Formation of ammonium salts with strong acids. • Reduction of nitro group to form amino group. (Formation of primary amine) • Reagent in nucleophilic substitution. • Formation of benzenediazonium salt from aniline with HNO2. • Reaction of benzenediazonium salt with various reagents to make substit ...
... • Formation of ammonium salts with strong acids. • Reduction of nitro group to form amino group. (Formation of primary amine) • Reagent in nucleophilic substitution. • Formation of benzenediazonium salt from aniline with HNO2. • Reaction of benzenediazonium salt with various reagents to make substit ...
Chapter 4
... (from the side of higher concentration of the ion to a side of lower ion concentration). ...
... (from the side of higher concentration of the ion to a side of lower ion concentration). ...
FUNCTIONAL GROUPS ORGANIC COMPOUNDS CONTAINING
... Strong non-covalent forces Exhibit higher melting and boiling points than alkanes ...
... Strong non-covalent forces Exhibit higher melting and boiling points than alkanes ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.