Organic molecules with functional groups containing oxygen
... organomagnesium reagents in synthesis. He was so successful that he was awarded the Nobel Prize for Chemistry in 1912. Today, the terms organomagnesium reagent and Grignard Reagent are used interchangeably. Grignard Reactions are important because they are a very good way of making the C-C bonds whi ...
... organomagnesium reagents in synthesis. He was so successful that he was awarded the Nobel Prize for Chemistry in 1912. Today, the terms organomagnesium reagent and Grignard Reagent are used interchangeably. Grignard Reactions are important because they are a very good way of making the C-C bonds whi ...
Chapter 1
... • Alcohols dehydrate with heat in the presence of strong acid to produce alkenes • Dehydration is a type of elimination reaction – A molecule loses atoms or ions from its structure – Here –OH and –H are removed / eliminate from adjacent carbon atoms to produce an alkene and water – A reversal of the ...
... • Alcohols dehydrate with heat in the presence of strong acid to produce alkenes • Dehydration is a type of elimination reaction – A molecule loses atoms or ions from its structure – Here –OH and –H are removed / eliminate from adjacent carbon atoms to produce an alkene and water – A reversal of the ...
File - Rasapalli Research Group
... Phenols are acidic in nature, and can be oxidized to quinones. 9. Alcohols upon reaction with HX provide alkyl halides. 10. An alkyl halide = an alkyl group with a halogen 11. Haloalkane Properties – Strongly affected by the C-X bond polarization and the polarizability of X. 12. Alcohols and alkyl h ...
... Phenols are acidic in nature, and can be oxidized to quinones. 9. Alcohols upon reaction with HX provide alkyl halides. 10. An alkyl halide = an alkyl group with a halogen 11. Haloalkane Properties – Strongly affected by the C-X bond polarization and the polarizability of X. 12. Alcohols and alkyl h ...
Types of reactions you know:
... Practicing Reactions: --A helpful resource is the companion website for the 6th edition of your textbook. Google “McMurrray Organic Chem” and it should be the first hit. I will also have a link on the SI website to the website. The “organic interactive” problems in each chapter are extremely helpfu ...
... Practicing Reactions: --A helpful resource is the companion website for the 6th edition of your textbook. Google “McMurrray Organic Chem” and it should be the first hit. I will also have a link on the SI website to the website. The “organic interactive” problems in each chapter are extremely helpfu ...
Enhanced diastereoselectivity of an ene hydroperoxidation reaction
... found that, in the intrazeolite photooxygenation of geminal dimethyl trisubstituted alkenes, formation of the new double bond in the ene adducts occurs preferentially at the methyl groups.2 Labeling experiments have shown that the ‘cis-effect’ selectivity found in solution does not operate within th ...
... found that, in the intrazeolite photooxygenation of geminal dimethyl trisubstituted alkenes, formation of the new double bond in the ene adducts occurs preferentially at the methyl groups.2 Labeling experiments have shown that the ‘cis-effect’ selectivity found in solution does not operate within th ...
Ch 22 Organic
... A group of atoms that determines an organic molecules’ chemical properties. It can take the place of a hydrogen in a hydrocarbon. ...
... A group of atoms that determines an organic molecules’ chemical properties. It can take the place of a hydrogen in a hydrocarbon. ...
Unit 2: Carbon Compounds
... A fuel is a chemical which is burned to produce energy. Combustion is another word for burning. When a substance burns it reacts with oxygen. The chemical compounds which are found in oil and natural gas are mainly hydrocarbons. A hydrocarbon is a compound which contains hydrogen and carbo ...
... A fuel is a chemical which is burned to produce energy. Combustion is another word for burning. When a substance burns it reacts with oxygen. The chemical compounds which are found in oil and natural gas are mainly hydrocarbons. A hydrocarbon is a compound which contains hydrogen and carbo ...
AS 2, Module 2
... (iii) If you had infrared spectra of the four butanols how would you use them to identify an unknown butanol? ...
... (iii) If you had infrared spectra of the four butanols how would you use them to identify an unknown butanol? ...
Unit 2: Carbon Compounds
... A fuel is a chemical which is burned to produce energy. Combustion is another word for burning. When a substance burns it reacts with oxygen. The chemical compounds which are found in oil and natural gas are mainly hydrocarbons. A hydrocarbon is a compound which contains hydrogen and carbo ...
... A fuel is a chemical which is burned to produce energy. Combustion is another word for burning. When a substance burns it reacts with oxygen. The chemical compounds which are found in oil and natural gas are mainly hydrocarbons. A hydrocarbon is a compound which contains hydrogen and carbo ...
Spring 2015 CH 421 Name ________________________________________ 1. Consider the structures of vanillin and vanillyl alcohol.
... 8. Which peak from the 1H NMR of vanillin will disappear upon its conversion to vanillyl alcohol? Indicate the chemical shift rounded to the tenths digit. (Note the other peaks may be shifted, but they are still present in the product.) ...
... 8. Which peak from the 1H NMR of vanillin will disappear upon its conversion to vanillyl alcohol? Indicate the chemical shift rounded to the tenths digit. (Note the other peaks may be shifted, but they are still present in the product.) ...
n - TU Chemnitz
... α-Azido alcohols derived from aldehydes are not known in literature but postulated to be unstable intermediates in solvolysis reactions. The synthesis starting from α-azidoalkyl trimethylsilyl ethers,[1] geminal diazides[2] or α-azido ethers[3] only led to the corresponding aldehydes but not to α-az ...
... α-Azido alcohols derived from aldehydes are not known in literature but postulated to be unstable intermediates in solvolysis reactions. The synthesis starting from α-azidoalkyl trimethylsilyl ethers,[1] geminal diazides[2] or α-azido ethers[3] only led to the corresponding aldehydes but not to α-az ...
Experiment 7 – Dehydration of Methylcyclohexanols
... • Elimination Reactions: Chapter 11.7 – 11.10 • Reactions of Alcohols (Dehydration): Chapter 17.6 • Oxidative Cleavage of Alkenes: Chapter 7.9 As the name suggests, dehydration reactions involve the loss of water. In organic synthesis, a dehydration reaction is synonymous with an elimination reactio ...
... • Elimination Reactions: Chapter 11.7 – 11.10 • Reactions of Alcohols (Dehydration): Chapter 17.6 • Oxidative Cleavage of Alkenes: Chapter 7.9 As the name suggests, dehydration reactions involve the loss of water. In organic synthesis, a dehydration reaction is synonymous with an elimination reactio ...
Chapter 17: Alcohols and Phenols
... Infrared (IR): Characteristic O–H stretching absorption at 3300 to 3600 cm−1 Sharp absorption near 3600 cm-1 except if H-bonded: then broad absorption 3300 to 3400 cm−1 range Strong C–O stretching absorption near 1050 cm−1 ...
... Infrared (IR): Characteristic O–H stretching absorption at 3300 to 3600 cm−1 Sharp absorption near 3600 cm-1 except if H-bonded: then broad absorption 3300 to 3400 cm−1 range Strong C–O stretching absorption near 1050 cm−1 ...
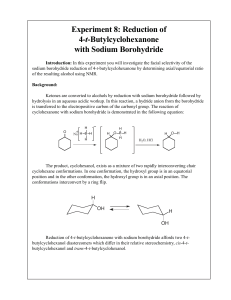
Experiment 8: Reduction of 4-t-Butylcyclohexanone with Sodium
... knowledge describes the behavior of water and most organic liquids, such as hexane, ether, and dichloromethane. Liquids that mix to form a homogeneous liquid phase are said to be miscible, meaning that the two substances are mutually soluble in all proportions. Most organic liquid used as solvents f ...
... knowledge describes the behavior of water and most organic liquids, such as hexane, ether, and dichloromethane. Liquids that mix to form a homogeneous liquid phase are said to be miscible, meaning that the two substances are mutually soluble in all proportions. Most organic liquid used as solvents f ...
Organic Chemistry Durham School Board March
... Organic compounds are an extremely diverse group of compounds with a variety of physical and chemical properties. In order to better understand these properties, chemists have organized organic compounds into useful groups called functional groups. Functional groups provide a useful way to classify ...
... Organic compounds are an extremely diverse group of compounds with a variety of physical and chemical properties. In order to better understand these properties, chemists have organized organic compounds into useful groups called functional groups. Functional groups provide a useful way to classify ...
xy3-allyl Benzoic Acid, CsHa(COOH)1(OW)2(CsH6)3.---Thi
... the chlorohydrine, the action then proceeding as above. As would be expected, the yield by this method is less than that by either of tbe other two. The advantages of the present syntheses are obvious. The materials used are all quite common and easily obtained, the reactions are simple and quickly ...
... the chlorohydrine, the action then proceeding as above. As would be expected, the yield by this method is less than that by either of tbe other two. The advantages of the present syntheses are obvious. The materials used are all quite common and easily obtained, the reactions are simple and quickly ...
Organic Chemistry
... Carbon is covalently bonded to another carbon, hydrogen and possibly to oxygen, a halogen or nitrogen Carbon joins other in chains or rings and can have branches coming off of these chains or rings One molecular formula can represent many different compounds (called structural isomers) Properties of ...
... Carbon is covalently bonded to another carbon, hydrogen and possibly to oxygen, a halogen or nitrogen Carbon joins other in chains or rings and can have branches coming off of these chains or rings One molecular formula can represent many different compounds (called structural isomers) Properties of ...
Unit 19 Chemistry Honors
... Unit 18, Chapter 20 Mrs. Frost 2012 www.hinsdale86.org/staff/kfrost Objectives: 1. Name and write formulas for organic compounds such as alkanes, alkenes, alkynes and molecules containing basic organic functional groups. 2. Draw organic structures. 3. Draw and identify the different types of isomers ...
... Unit 18, Chapter 20 Mrs. Frost 2012 www.hinsdale86.org/staff/kfrost Objectives: 1. Name and write formulas for organic compounds such as alkanes, alkenes, alkynes and molecules containing basic organic functional groups. 2. Draw organic structures. 3. Draw and identify the different types of isomers ...
Elias lecture chemistry of chlorine 2016 nov
... •Chlorine has the highest electron affinity (348.6 kJ/mol) and the third highest electronegativity (3.16) of all the reactive elements. The Cl-Cl bond dissociation energy (58 kcal/mol) is the highest among dihalogen molecules. •Chlorine gas and many of its compounds are chemicals useful in water pu ...
... •Chlorine has the highest electron affinity (348.6 kJ/mol) and the third highest electronegativity (3.16) of all the reactive elements. The Cl-Cl bond dissociation energy (58 kcal/mol) is the highest among dihalogen molecules. •Chlorine gas and many of its compounds are chemicals useful in water pu ...
Organic Molecules: Introduction and key concepts
... structures containing thousands of atoms! Although carbon is present in all organic compounds, other elements such as hydrogen (H), oxygen (O), nitrogen (N), sulfur (S) and phosphorus (P) are also common in these molecules. Until the early nineteenth century, chemists had managed to make many simple ...
... structures containing thousands of atoms! Although carbon is present in all organic compounds, other elements such as hydrogen (H), oxygen (O), nitrogen (N), sulfur (S) and phosphorus (P) are also common in these molecules. Until the early nineteenth century, chemists had managed to make many simple ...
The alkenes
... CHEMICAL PROPERTIES OF ALKENES ELECTROPHILIC ADDITION MECHANISM The electrophile, having some positive character is attracted to the alkene. The electrons in the pi bond come out to form a bond to the positive end. Because hydrogen can only have two electrons in its orbital, its other bond breaks h ...
... CHEMICAL PROPERTIES OF ALKENES ELECTROPHILIC ADDITION MECHANISM The electrophile, having some positive character is attracted to the alkene. The electrons in the pi bond come out to form a bond to the positive end. Because hydrogen can only have two electrons in its orbital, its other bond breaks h ...
Nucleophilic Substitution and b
... • RO-, an alkoxide ion, is both a strong nucleophile (unless bulky and hindered) and a strong base. Both SN2 (desired) and E2 (undesired side product) can occur. • Choose nucleophile and electrophile carefully. Maximize SN2 and minimize E2 reaction by choosing the R’X to have least substituted carbo ...
... • RO-, an alkoxide ion, is both a strong nucleophile (unless bulky and hindered) and a strong base. Both SN2 (desired) and E2 (undesired side product) can occur. • Choose nucleophile and electrophile carefully. Maximize SN2 and minimize E2 reaction by choosing the R’X to have least substituted carbo ...
OS-FGI Lecture2
... Singlet oxygen (1O2) is the first excited electronic state of molecular oxygen, and it is VERY reactive! It can be generated by photolysing oxygen in the presence of a photosensitiser (usually a dye such as Rose Bengal). Singlet oxygen is nicely complementary to SeO2 in its allylic oxidations in tha ...
... Singlet oxygen (1O2) is the first excited electronic state of molecular oxygen, and it is VERY reactive! It can be generated by photolysing oxygen in the presence of a photosensitiser (usually a dye such as Rose Bengal). Singlet oxygen is nicely complementary to SeO2 in its allylic oxidations in tha ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.