幻灯片 1
... Bonding in organic compounds at that time was thought to be of either the water type, as in alcohols, ROH, or of the radical type, as in ethers which would be given the formula RO. But Williamson, by his ether synthesis, showed that mixed ethers, with two different alkyl groups, could be prepared. ...
... Bonding in organic compounds at that time was thought to be of either the water type, as in alcohols, ROH, or of the radical type, as in ethers which would be given the formula RO. But Williamson, by his ether synthesis, showed that mixed ethers, with two different alkyl groups, could be prepared. ...
Reactions of carbon radicals generated by 1,5
... sors of alkoxyl radicals, and can be prepared in a separate reaction and then subjected to photolytic decomposition.14 Among the most convenient and easily available precursors of alkoxyl radicals are the alkyl nitrites 4b (RO-NO, 222, kJ/mol), prepared by the esterification of alcohols with nitrous ...
... sors of alkoxyl radicals, and can be prepared in a separate reaction and then subjected to photolytic decomposition.14 Among the most convenient and easily available precursors of alkoxyl radicals are the alkyl nitrites 4b (RO-NO, 222, kJ/mol), prepared by the esterification of alcohols with nitrous ...
Organic Chemistry Notes by Jim Maxka jim.maxka
... In general, we use P, S, and Si reagents with C-O because, the formation of the P-O, S-O, and Si-O bond is more stabilizing than the C-O. That means that there is a thermodynamic driving force for the C-O bond to be broken. 10 alcohols: To eliminate a 10 alcohol, we really have to heat things up, be ...
... In general, we use P, S, and Si reagents with C-O because, the formation of the P-O, S-O, and Si-O bond is more stabilizing than the C-O. That means that there is a thermodynamic driving force for the C-O bond to be broken. 10 alcohols: To eliminate a 10 alcohol, we really have to heat things up, be ...
Selective Oxidation Reactions of Natural Compounds with
... Oxyfunctionalization of cheap natural compounds is a useful protocol to obtain molecules widely employed in the fine chemicals-based industries as fragrances, flavors, and therapeutically active substances [1]. The most commonly employed stoichiometric oxidants are organic peroxyacids, particularly ...
... Oxyfunctionalization of cheap natural compounds is a useful protocol to obtain molecules widely employed in the fine chemicals-based industries as fragrances, flavors, and therapeutically active substances [1]. The most commonly employed stoichiometric oxidants are organic peroxyacids, particularly ...
Chapter 17 Notes
... C = C bonds undergo addition reactions C = O bonds also undergo addition reactions positive species are attracted to the oxygen negative species are attracted to the carbon Addition of alcohols to aldehydes O ...
... C = C bonds undergo addition reactions C = O bonds also undergo addition reactions positive species are attracted to the oxygen negative species are attracted to the carbon Addition of alcohols to aldehydes O ...
Organic Chemistry
... bonds in the same way that they add to carbonoxygen double bonds. The product of the reaction is an imine. ...
... bonds in the same way that they add to carbonoxygen double bonds. The product of the reaction is an imine. ...
Organic Chemistry Introduction
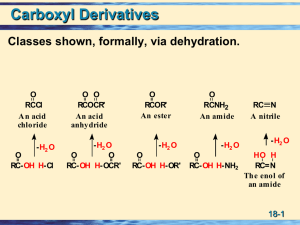
... • Acylation of Aromatic Rings • Reaction of an acid chloride (RCOCl) with an aromatic ring in the presence of AlCl3 introduces the acyl group, ...
... • Acylation of Aromatic Rings • Reaction of an acid chloride (RCOCl) with an aromatic ring in the presence of AlCl3 introduces the acyl group, ...
CHM-373 American Women in Science and Society
... Synthesis of Aldehydes and Ketones • Hydration of Alkynes • Involves a keto-enol tautomerization • Mixture of ketones seen with internal alkynes ...
... Synthesis of Aldehydes and Ketones • Hydration of Alkynes • Involves a keto-enol tautomerization • Mixture of ketones seen with internal alkynes ...
Chemistry 11 – Functional Groups Notes
... parent name of the compound. 2. Change the ending of the parent name to the ending specific to the functional group. 3. Number the parent chain from the end nearest to the functional group so that the functional group gets the lowest possible number. Place this number, along with a dash in front of ...
... parent name of the compound. 2. Change the ending of the parent name to the ending specific to the functional group. 3. Number the parent chain from the end nearest to the functional group so that the functional group gets the lowest possible number. Place this number, along with a dash in front of ...
4797 Chem Test Ch 21
... MULTIPLE CHOICE On the line at the left of each statement, write the letter of the choice that best completes the statement or answers the question. ...
... MULTIPLE CHOICE On the line at the left of each statement, write the letter of the choice that best completes the statement or answers the question. ...
Organic Chemistry II
... cyclohexene, which has a characteristic odor, you are losing product and your percent yield will be lower. Heat the mixture and note the temperature as the distillation proceeds. You may need to wrap the fractionating column in aluminum foil so that the hot vapors do not become too cool to distill o ...
... cyclohexene, which has a characteristic odor, you are losing product and your percent yield will be lower. Heat the mixture and note the temperature as the distillation proceeds. You may need to wrap the fractionating column in aluminum foil so that the hot vapors do not become too cool to distill o ...
Organic Chemistry
... • they are tools with which to search for new information and new understanding ...
... • they are tools with which to search for new information and new understanding ...
Second Year - WordPress.com
... a) Atomic weight of any one element was found to be approximately the mean of the other two elements of triad. b) Atomic weight of the middle element was found to be approximately the mean of the other two elements of a triad. c) Atomic number of any one element was found to be approximately the mea ...
... a) Atomic weight of any one element was found to be approximately the mean of the other two elements of triad. b) Atomic weight of the middle element was found to be approximately the mean of the other two elements of a triad. c) Atomic number of any one element was found to be approximately the mea ...
bonding
... X from adjacent carbons of an alkyl halide to form an alkene. Elimination of alkyl halides is affected by base, often the conjugate bases of alcohols (alkoxides), by an E2 mechanism The reaction follows Zaitsev's rule in that the most stable double bond product usually predominates H3CH2CO - Na+, H3 ...
... X from adjacent carbons of an alkyl halide to form an alkene. Elimination of alkyl halides is affected by base, often the conjugate bases of alcohols (alkoxides), by an E2 mechanism The reaction follows Zaitsev's rule in that the most stable double bond product usually predominates H3CH2CO - Na+, H3 ...
A Convenient Preparation of Volatile Acid Chlorides
... rides for the preparation of other acid chlorides distilled through a small column directly out of the has been neglected for the most part. Adams and reaction mixture. The reaction is general, limUlichl have reported that they could obtain al- ited only by the volatility of the acid chloridemost qu ...
... rides for the preparation of other acid chlorides distilled through a small column directly out of the has been neglected for the most part. Adams and reaction mixture. The reaction is general, limUlichl have reported that they could obtain al- ited only by the volatility of the acid chloridemost qu ...
14. The Direct and Enantioselective Organocatalytic -Oxidation of Aldehydes
... and 6) is possible without loss in efficiency or enantiocontrol (6088% yield, 97-99% ee). Notably, these mild reaction conditions allow the use of electron-rich π-systems which are typically prone to oxidative degradation. For example, enamine oxidation to access enantio-enriched R-oxyaldehydes can ...
... and 6) is possible without loss in efficiency or enantiocontrol (6088% yield, 97-99% ee). Notably, these mild reaction conditions allow the use of electron-rich π-systems which are typically prone to oxidative degradation. For example, enamine oxidation to access enantio-enriched R-oxyaldehydes can ...
Name Reactions in Heterocyclic Chemistry-II
... Detailed procedures were published for synthesizing 2,4,6-trimethylpyrylium perchlorate,42 tetrafluoroborate,43 triflate,44 and sulfoacetate.45 Being salts, these compounds are insoluble in ether and are therefore easily purified from side-products such as mesityl oxide, so that yields are at least ...
... Detailed procedures were published for synthesizing 2,4,6-trimethylpyrylium perchlorate,42 tetrafluoroborate,43 triflate,44 and sulfoacetate.45 Being salts, these compounds are insoluble in ether and are therefore easily purified from side-products such as mesityl oxide, so that yields are at least ...
Aromatic Hydrocarbon Tutorial
... intermolecular interactions with H2O and other polar compounds, aromatic hydrocarbons are considered to be insoluble in these media. Remember, water is a polar (H-O-H) substance that forms an ordered medium characterized by a high degree of intermolecular H-bonding. To dissolve in water, a “solute” ...
... intermolecular interactions with H2O and other polar compounds, aromatic hydrocarbons are considered to be insoluble in these media. Remember, water is a polar (H-O-H) substance that forms an ordered medium characterized by a high degree of intermolecular H-bonding. To dissolve in water, a “solute” ...
102 Lecture Ch15
... • Because the carbonyl group is polar, aldehydes and ketones have higher boiling points than hydrocarbons • However, they have no H attached to the O, so do not have hydrogen bonding, and have lower boiling points than alcohols • Like ethers, aldehydes and ketones can hydrogen bond with water, so th ...
... • Because the carbonyl group is polar, aldehydes and ketones have higher boiling points than hydrocarbons • However, they have no H attached to the O, so do not have hydrogen bonding, and have lower boiling points than alcohols • Like ethers, aldehydes and ketones can hydrogen bond with water, so th ...
Stockholm University
... The allylation of imine 4 substrates also starts with palladium-catalyzed formation of the allylboronate 11. In contrast to aldehyde 3, imine 4 is not able to undergo direct electrophilic substitution with 11 (Scheme 7), and therefore the allylation step requires palladium catalysis. The second cata ...
... The allylation of imine 4 substrates also starts with palladium-catalyzed formation of the allylboronate 11. In contrast to aldehyde 3, imine 4 is not able to undergo direct electrophilic substitution with 11 (Scheme 7), and therefore the allylation step requires palladium catalysis. The second cata ...
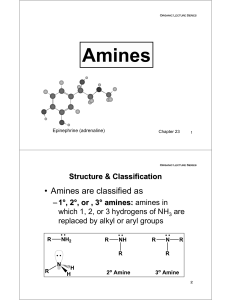
Amines
... • Aliphatic diazonium ions are unstable and lose N2 to give a carbocation which may 1. lose a proton to give an alkene 2. react with a nucleophile to give a substitution product 3. rearrange and then react by 1 and/or 2 Cl ...
... • Aliphatic diazonium ions are unstable and lose N2 to give a carbocation which may 1. lose a proton to give an alkene 2. react with a nucleophile to give a substitution product 3. rearrange and then react by 1 and/or 2 Cl ...
Drawing Organic Structures Functional Groups Constitutional Isomers
... • Odd masses have 1 or 3 nitrogen atoms • Even masses have 0 or 2 nitrogen atoms ...
... • Odd masses have 1 or 3 nitrogen atoms • Even masses have 0 or 2 nitrogen atoms ...
Mechanistic Studies on Alcoholysis of α-Keto esters
... According to the consecutive mechanism, the methanolysis does not occur without going through the hemiacetal intermediate, or classical methanolysis of ester portion occurs at much slower rate than the methanolysis via the hemiacetal does. Thus, we have prepared two sterically hindered α-keto esters ...
... According to the consecutive mechanism, the methanolysis does not occur without going through the hemiacetal intermediate, or classical methanolysis of ester portion occurs at much slower rate than the methanolysis via the hemiacetal does. Thus, we have prepared two sterically hindered α-keto esters ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.