reactions of the conjugated dienes butadiene and isoprene alone
... that the reactions often take place under somewhat milder conditions than are usually employed (see, e.g., Adams et al., 1982c; Ballantine et al., 1981a). The reactions of monoalkenes over acidic montmorillonite catalysts have been extensively studied, both alone and in the presence ofnucleophiles, ...
... that the reactions often take place under somewhat milder conditions than are usually employed (see, e.g., Adams et al., 1982c; Ballantine et al., 1981a). The reactions of monoalkenes over acidic montmorillonite catalysts have been extensively studied, both alone and in the presence ofnucleophiles, ...
Ch 19 Aldehydes and Ketones
... Aldehydes (RCHO), with the exception of formaldehyde (H2CO), are compounds with both an H and an organic group attached to a carbonyl. Ketones (R2CO) are compounds with two organic groups attached to a carbonyl. Naming Aldehydes 1. Replace final “e” of alkane parent with “al”, such as ethanal (aceta ...
... Aldehydes (RCHO), with the exception of formaldehyde (H2CO), are compounds with both an H and an organic group attached to a carbonyl. Ketones (R2CO) are compounds with two organic groups attached to a carbonyl. Naming Aldehydes 1. Replace final “e” of alkane parent with “al”, such as ethanal (aceta ...
Chapter 1 Organoaluminum Reagents for Selective Organic
... classical Lewis acids, the steric effect of our aluminum reagents also plays an important role in selective organic synthesis [R-27, 28, 323]. Thus, MAD, ATPH, methylaluminum bis(4-bromo-2,6-di-tert-butylphenoxide) (MABR) and methylaluminum bis(2,6-diphenylphenoxide)(MAPH) are readily prepared by tr ...
... classical Lewis acids, the steric effect of our aluminum reagents also plays an important role in selective organic synthesis [R-27, 28, 323]. Thus, MAD, ATPH, methylaluminum bis(4-bromo-2,6-di-tert-butylphenoxide) (MABR) and methylaluminum bis(2,6-diphenylphenoxide)(MAPH) are readily prepared by tr ...
E- Sedatives and Hypnotics Lectures
... Cyclobarbital is a short acting hypnotic, with 1-cyclohexenyl group at position 5. The normal synthetic route for barbiturates is not applicable for such derivative, it s prepared as follows: ...
... Cyclobarbital is a short acting hypnotic, with 1-cyclohexenyl group at position 5. The normal synthetic route for barbiturates is not applicable for such derivative, it s prepared as follows: ...
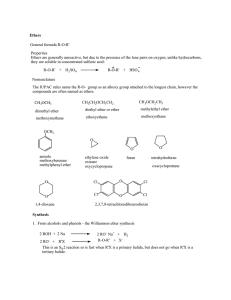
Ethers General formula R-O-R` Properties Ethers are generally
... The IUPAC rules name the R-O- group as an alkoxy group attached to the longest chain, however the compounds are often named as ethers. CH3OCH3 ...
... The IUPAC rules name the R-O- group as an alkoxy group attached to the longest chain, however the compounds are often named as ethers. CH3OCH3 ...
physicochemical properties of organic medicinal agents
... dipoles (C-O-C), and thus the carbon atoms attached to oxygen are somewhat electrophilic in nature (compared to a typical hydrocarbon C-C or C-H carbon). These dipoles are relatively weak compared to dipoles such as the carbonyl found in esters and amides (see Ester and Amide Tutorials). Thus ethers ...
... dipoles (C-O-C), and thus the carbon atoms attached to oxygen are somewhat electrophilic in nature (compared to a typical hydrocarbon C-C or C-H carbon). These dipoles are relatively weak compared to dipoles such as the carbonyl found in esters and amides (see Ester and Amide Tutorials). Thus ethers ...
Stoichiometry of Ozonation of Environmentally
... carboxylic acids to form alkoxy-, hydroxy-, and acyloxyhydroperoxides, respectively, and isomerization into carboxylic acids (16). In gas-phase ozonation reactions, carbonyl oxides may also undergo unimolecular decomposition to give OH radicals in fairly high yields (17). It is often assumed that oz ...
... carboxylic acids to form alkoxy-, hydroxy-, and acyloxyhydroperoxides, respectively, and isomerization into carboxylic acids (16). In gas-phase ozonation reactions, carbonyl oxides may also undergo unimolecular decomposition to give OH radicals in fairly high yields (17). It is often assumed that oz ...
Reactions of Aldehydes and Ketones – Nucleophilic Addition
... B5. The Wittig Reaction The phosphorus-containing reagent is called a phosphorane, and it belongs to a larger class of compounds called ylides. An ylide is a compound with two oppositely charged atoms adjacent to each other. ...
... B5. The Wittig Reaction The phosphorus-containing reagent is called a phosphorane, and it belongs to a larger class of compounds called ylides. An ylide is a compound with two oppositely charged atoms adjacent to each other. ...
Alcohols - La Salle University
... • Women will have higher BAL’s with the consumption of an equal number of drinks due to lower ADH activity and lower % H2O in blood. • Estradiol levels increase in women (and men). This has been associated with higher incidences of heart disease and a change in bone density. • A higher than normal c ...
... • Women will have higher BAL’s with the consumption of an equal number of drinks due to lower ADH activity and lower % H2O in blood. • Estradiol levels increase in women (and men). This has been associated with higher incidences of heart disease and a change in bone density. • A higher than normal c ...
Chapter 16 - Chemistry of Benzene
... reactions with Lewis acids The product is formed by loss of a proton, which is replaced by bromine FeBr3 is added as a catalyst to polarize the bromine ...
... reactions with Lewis acids The product is formed by loss of a proton, which is replaced by bromine FeBr3 is added as a catalyst to polarize the bromine ...
Electophilic Aromatic Substituion
... reactions with Lewis acids The product is formed by loss of a proton, which is replaced by bromine FeBr3 is added as a catalyst to polarize the bromine ...
... reactions with Lewis acids The product is formed by loss of a proton, which is replaced by bromine FeBr3 is added as a catalyst to polarize the bromine ...
A mechanistic approach to solvolysis of n-caproyl chloride (n
... The study of the rate and rate constants in the alcoholysis of n-caproyl chloride in acetone and acetonitrile, reveals that SN2 mechanism is more favourable than SN1. A plot of logarithm of rate constant against the logarithm of molar concentration of alcohol gives a straight line with a slope close ...
... The study of the rate and rate constants in the alcoholysis of n-caproyl chloride in acetone and acetonitrile, reveals that SN2 mechanism is more favourable than SN1. A plot of logarithm of rate constant against the logarithm of molar concentration of alcohol gives a straight line with a slope close ...
Triruthenium and triosmium carbonyl clusters containing chiral
... the reaction pathway that leads to the mixture of 1 and 2 from [Ru3 (CO)12 ] and levamisolium chloride, we treated the activated cluster [Ru3 (CO)10 (MeCN)2 ] with levamisolium chloride in dichloromethane at room temperature ([Ru3 (CO)12 ] does not react with levamisolium chloride at this temperatur ...
... the reaction pathway that leads to the mixture of 1 and 2 from [Ru3 (CO)12 ] and levamisolium chloride, we treated the activated cluster [Ru3 (CO)10 (MeCN)2 ] with levamisolium chloride in dichloromethane at room temperature ([Ru3 (CO)12 ] does not react with levamisolium chloride at this temperatur ...
Lecture Notes_ch17
... • Identify the parent chain: the longest chain of carbons to which the nitrogen is attached • Replace the “-e” ending of the alkane name with “amine” • Number the parent chain from the end closest to the nitrogen atom • Identify the position of the nitrogen atom with a ...
... • Identify the parent chain: the longest chain of carbons to which the nitrogen is attached • Replace the “-e” ending of the alkane name with “amine” • Number the parent chain from the end closest to the nitrogen atom • Identify the position of the nitrogen atom with a ...
laman web smk raja perempuan, ipoh
... hydrolysis of bromoethane, the formation of nitriles, and the formation of primary amines by reaction with NH3 (b) haloalkane elimination reactions which produce alkenes 2. explain the mechanism of nucleophilic substitution in haloalkanes 3. explain the difference in the reactivity of chlorobenzene ...
... hydrolysis of bromoethane, the formation of nitriles, and the formation of primary amines by reaction with NH3 (b) haloalkane elimination reactions which produce alkenes 2. explain the mechanism of nucleophilic substitution in haloalkanes 3. explain the difference in the reactivity of chlorobenzene ...
4.7 Organic chemistry
... This resource provides guidance for teaching the topic Organic chemistry from our new GCSE Chemistry (8462). It has been updated from the draft version to reflect the changes made in the accredited specification. These changes are also reflected in the learning outcomes and opportunities to develop ...
... This resource provides guidance for teaching the topic Organic chemistry from our new GCSE Chemistry (8462). It has been updated from the draft version to reflect the changes made in the accredited specification. These changes are also reflected in the learning outcomes and opportunities to develop ...
10.4 Alcohols - SCIS Teachers
... •Alcohols have the general formula: CnH2n+1OH •The physical properties of alcohols are similar to those of both water and hydrocarbons •The shorter chain alcohols such as methanol and ethanol are similar to water, in general they •have higher boiling points than hydrocarbons but lower than water •di ...
... •Alcohols have the general formula: CnH2n+1OH •The physical properties of alcohols are similar to those of both water and hydrocarbons •The shorter chain alcohols such as methanol and ethanol are similar to water, in general they •have higher boiling points than hydrocarbons but lower than water •di ...
Highly Enantioselective Cyclocarbonylation of Allylic
... chiral γ-butyrolactones 4 in good to excellent enantioselectivities (up to 98% ee) (Table 2). In general, introduction of aromatic substituents at the β or γ position of allylic alcohols enhances the enantioselectivity (entries 1-6 vs entry 7). The lactonization of the allylic alcohols 3 likely proc ...
... chiral γ-butyrolactones 4 in good to excellent enantioselectivities (up to 98% ee) (Table 2). In general, introduction of aromatic substituents at the β or γ position of allylic alcohols enhances the enantioselectivity (entries 1-6 vs entry 7). The lactonization of the allylic alcohols 3 likely proc ...
120 Chapter 24: Phenols. Alcohols contain an OH group bonded to
... tosylates afford aryl ethers (Williamson ether synthesis) OH ...
... tosylates afford aryl ethers (Williamson ether synthesis) OH ...
english,
... surface), water soluble transport forms of volatile apolar compounds and some of them posses biological activity. ...
... surface), water soluble transport forms of volatile apolar compounds and some of them posses biological activity. ...
投影片 1
... Tertiary alcohol (e.g. t-butyl) urethanes are relatively unstable and may thermally decompose to give alkenes, carbon dioxide, and amines. ...
... Tertiary alcohol (e.g. t-butyl) urethanes are relatively unstable and may thermally decompose to give alkenes, carbon dioxide, and amines. ...
Iron(II) Chloride–1,1′-Binaphthyl-2,2′-diamine
... substrates. Hence, there is a need for an efficient, economic and ecofriendly catalyst for the synthesis of 1,1-bisindolylmethanes starting from primary alcohols. Iron is an attractive alternative catalyst because of its abundance, low price and environmentally benign character.21 Unlike other metal ...
... substrates. Hence, there is a need for an efficient, economic and ecofriendly catalyst for the synthesis of 1,1-bisindolylmethanes starting from primary alcohols. Iron is an attractive alternative catalyst because of its abundance, low price and environmentally benign character.21 Unlike other metal ...
CHEM 203 Material
... Example: the C atom in CH4 has formally acquired 4 electrons, thereby assuming the oxidation state of –4. This produces a significant concentration of electronic density around the C atom. One may predict that the C atom in methane will behave as an electron donor in its reactions; that is, it will ...
... Example: the C atom in CH4 has formally acquired 4 electrons, thereby assuming the oxidation state of –4. This produces a significant concentration of electronic density around the C atom. One may predict that the C atom in methane will behave as an electron donor in its reactions; that is, it will ...
Organic Chemistry II Introduction
... ArOH is more acidic than ROH Soluble in dilute NaOH Anion is resonance stabilized EWG make phenols more acidic than phenol EDG make phenols less acidic than phenol O ...
... ArOH is more acidic than ROH Soluble in dilute NaOH Anion is resonance stabilized EWG make phenols more acidic than phenol EDG make phenols less acidic than phenol O ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.