Chem 2423-Test 2 - HCC Learning Web
... Oxygen does not affect the base formula. A hydrogen is added to the base formula for each halogen and subtracted for each nitrogen so the base formula for diazepam is C16H12. The saturated 16 carbon compound would have 34 hydrogens so the number of degrees of unsaturation for diazepam is: (34 − 12) ...
... Oxygen does not affect the base formula. A hydrogen is added to the base formula for each halogen and subtracted for each nitrogen so the base formula for diazepam is C16H12. The saturated 16 carbon compound would have 34 hydrogens so the number of degrees of unsaturation for diazepam is: (34 − 12) ...
Carboxylic Acids and Their Derivatives
... Reactions of Acids-4 Acids form salts with bases. The salts react with strong mineral acids to give the original organic acid. O O CH3C OH + NaOH CH3C ONa + H2O O O CH3C OH + NaHCO3 CH3C ONa + H2O + CO2 ...
... Reactions of Acids-4 Acids form salts with bases. The salts react with strong mineral acids to give the original organic acid. O O CH3C OH + NaOH CH3C ONa + H2O O O CH3C OH + NaHCO3 CH3C ONa + H2O + CO2 ...
In the bachelor thesis of Esther Schippers, research is
... therefore also be soluble in aqueous solvents. To achieve this, for instance an ionic charge can be brought in the molecule. Ions are often good soluble in aqueous solutions. 3. The scaffold should have a part of the molecule that can easily be synthesized with another functional group. When differe ...
... therefore also be soluble in aqueous solvents. To achieve this, for instance an ionic charge can be brought in the molecule. Ions are often good soluble in aqueous solutions. 3. The scaffold should have a part of the molecule that can easily be synthesized with another functional group. When differe ...
m4 phenol and diazo salts
... Sodium phenol reacts with sodium to form an ionic salt - sodium phenoxide hydrogen is also produced this reaction is similar to that with aliphatic alcohols such as ethanol 2C6H5OH(s) ...
... Sodium phenol reacts with sodium to form an ionic salt - sodium phenoxide hydrogen is also produced this reaction is similar to that with aliphatic alcohols such as ethanol 2C6H5OH(s) ...
Carboxylic Acid Derivatives and Nucleophilic Acyl Substitution
... Carbonyl carbon least electrophilic ...
... Carbonyl carbon least electrophilic ...
Contents - Personal WWW Pages
... Let us consider these advantages and disadvantages in a little more detail. Activity and Selectivity: these often have an inverse relation in both homogeneous and heterogeneous catalysis. i.e. faster reactions are often less selective. So, although homogeneous catalysis has a major advantage in the ...
... Let us consider these advantages and disadvantages in a little more detail. Activity and Selectivity: these often have an inverse relation in both homogeneous and heterogeneous catalysis. i.e. faster reactions are often less selective. So, although homogeneous catalysis has a major advantage in the ...
full size
... group (methanal, the simplest, has two). ¾The group is somewhat more polar than ethers, but like ethers it cannot donate a hydrogen bond to itself. Thus aldehydes are less volatile (higher boiling) than alkanes or ethers but are more volatile than alcohols or carboxylic acids. They are slightly less ...
... group (methanal, the simplest, has two). ¾The group is somewhat more polar than ethers, but like ethers it cannot donate a hydrogen bond to itself. Thus aldehydes are less volatile (higher boiling) than alkanes or ethers but are more volatile than alcohols or carboxylic acids. They are slightly less ...
Esters from Carboxylic Acid Anhydrides
... Leaving group ability is inversely related to basicity Chloride is the weakest base and the best leaving group Amines are the strongest bases and the worst leaving groups ...
... Leaving group ability is inversely related to basicity Chloride is the weakest base and the best leaving group Amines are the strongest bases and the worst leaving groups ...
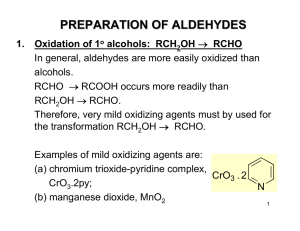
PREPARATION OF ALDEHYDES
... EXAMPLES OF NUCLEOPHILIC ADDITION TO ALDEHYDES & KETONES Addition of HCN (neutral-basic conditions). CN Ө is a very good nucleophile (ionic nucleophile). The use of the actual compound HCN is not experimentally feasible, as it is a lethal gas, bp 26 oC. Addition of the elements of HCN to a C=O grou ...
... EXAMPLES OF NUCLEOPHILIC ADDITION TO ALDEHYDES & KETONES Addition of HCN (neutral-basic conditions). CN Ө is a very good nucleophile (ionic nucleophile). The use of the actual compound HCN is not experimentally feasible, as it is a lethal gas, bp 26 oC. Addition of the elements of HCN to a C=O grou ...
Chemistry - Tiwariacademy.net
... Crystallisation is one of the most commonly used techniques for the purification of solid organic compounds. Principle: It is based on the difference in the solubilites of the compound and the impurities in a given solvent. The impure compound gets dissolved in the solvent in which it is sparingly s ...
... Crystallisation is one of the most commonly used techniques for the purification of solid organic compounds. Principle: It is based on the difference in the solubilites of the compound and the impurities in a given solvent. The impure compound gets dissolved in the solvent in which it is sparingly s ...
aldehydes and ketones
... • Because of the polarity of the C=O group, these groups can interact, but the attraction is not as strong as hydrogen bonding. • This makes the boiling point of aldehydes and ketones higher than alkanes, but lower than alcohols. ...
... • Because of the polarity of the C=O group, these groups can interact, but the attraction is not as strong as hydrogen bonding. • This makes the boiling point of aldehydes and ketones higher than alkanes, but lower than alcohols. ...
226 amines lec
... Effect of Resonance on Basicity — Aromatic amines are less basic than aliphatic amines, mostly because of resonance. The aromatic amine is more stabilized compared to its ammonium ion than an aliphatic amine is compared to its ammonium ion — ...
... Effect of Resonance on Basicity — Aromatic amines are less basic than aliphatic amines, mostly because of resonance. The aromatic amine is more stabilized compared to its ammonium ion than an aliphatic amine is compared to its ammonium ion — ...
EASTERN ARIZONA COLLEGE General Organic Chemistry I
... Students will gain an understanding of the role that organic chemistry plays in their lives, and the role that organic chemistry plays in the agricultural and medical fields. Students learn how to identify problems and work as a team to solve those problems. Students learn how to predict reactions a ...
... Students will gain an understanding of the role that organic chemistry plays in their lives, and the role that organic chemistry plays in the agricultural and medical fields. Students learn how to identify problems and work as a team to solve those problems. Students learn how to predict reactions a ...
proline catalyzed direct asymmetric aldol and mannich reactions
... Throughout the development of catalytic asymmetric organic synthetic methods, researchers have focused primarily on metal-mediated catalysis. Metal complexes have been shown to catalyze a wide variety of transformations stereoselectively; however, many catalytic metal complexes are difficult to remo ...
... Throughout the development of catalytic asymmetric organic synthetic methods, researchers have focused primarily on metal-mediated catalysis. Metal complexes have been shown to catalyze a wide variety of transformations stereoselectively; however, many catalytic metal complexes are difficult to remo ...
4. Amines Amides and Amino Acids
... This is a similar ligand exchange reaction to the one where ammonia acts as the ligand 4NH3 + Cu(H2O)62+ ...
... This is a similar ligand exchange reaction to the one where ammonia acts as the ligand 4NH3 + Cu(H2O)62+ ...
Aldehydes, Ketones and Carboxylic Acids
... Unlike ketones, aldehydes can be easily oxidised to carboxylic acids using a variety of oxidising agents. These reagents can be chromic acid, chromium trioxide, permanaganate or silver oxide. You have already read about oxidation with some of these reagents. Silver ions selectively oxidise —CHO grou ...
... Unlike ketones, aldehydes can be easily oxidised to carboxylic acids using a variety of oxidising agents. These reagents can be chromic acid, chromium trioxide, permanaganate or silver oxide. You have already read about oxidation with some of these reagents. Silver ions selectively oxidise —CHO grou ...
Carbonyl Chemistry (12 Lectures) Aldehydes and Ketones
... Formation of alcohols via addition of Grignard reagents to aldehydes and ketones is carried out in two separate steps Step 1: Addition of the nucleophilic alkyl group to the carbonyl carbon, aided by Lewis acid interaction between MgX+ and the carbonyl oxygen. The product of this step is a halomagne ...
... Formation of alcohols via addition of Grignard reagents to aldehydes and ketones is carried out in two separate steps Step 1: Addition of the nucleophilic alkyl group to the carbonyl carbon, aided by Lewis acid interaction between MgX+ and the carbonyl oxygen. The product of this step is a halomagne ...
Alcohols, Aldehydes, and Ketones
... of a primary alcohol at the aldehyde stage in order to prevent the aldehyde from being oxidized further to the carboxylic acid. One way to do this is to remove the aldehyde as soon as it is formed by distilling it from the reaction mixture. Reactions such as these can be used to measure alcohol cont ...
... of a primary alcohol at the aldehyde stage in order to prevent the aldehyde from being oxidized further to the carboxylic acid. One way to do this is to remove the aldehyde as soon as it is formed by distilling it from the reaction mixture. Reactions such as these can be used to measure alcohol cont ...
Mechanisms of Alkenes
... Formation of 3º halides: Steps Involved: – Tertiary OH must turn into a better leaving group by picking up a H+ to form water – Loss of H2O and Carbocation formation – Attack of halide to form tertiary halide ...
... Formation of 3º halides: Steps Involved: – Tertiary OH must turn into a better leaving group by picking up a H+ to form water – Loss of H2O and Carbocation formation – Attack of halide to form tertiary halide ...
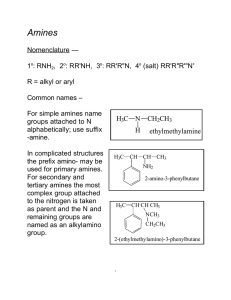
Amines: The Basic Group
... Amines, like alcohols, can be put into classes. Class depends on the number of carbons directly bonded to the nitrogen atom: a primary amine has one bond, a secondary two, etc. ...
... Amines, like alcohols, can be put into classes. Class depends on the number of carbons directly bonded to the nitrogen atom: a primary amine has one bond, a secondary two, etc. ...
Carboxylic Acids and Nitriles
... Hydrolysis: Conversion of Nitriles into Carboxylic Acids A nitrile can be hydrolyzed in either basic or acidic aqueous solution to yield a carboxylic acid and ammoniz or an amine O ...
... Hydrolysis: Conversion of Nitriles into Carboxylic Acids A nitrile can be hydrolyzed in either basic or acidic aqueous solution to yield a carboxylic acid and ammoniz or an amine O ...
Chapter 25 Organic and Biological Chemistry
... • Aromatic hydrocarbons are cyclic hydrocarbons that have some particular features. • There is a p-orbital on each atom. – The molecule is planar. ...
... • Aromatic hydrocarbons are cyclic hydrocarbons that have some particular features. • There is a p-orbital on each atom. – The molecule is planar. ...
Ether, Epoxides and Thiols
... An ether has two organic groups (alkyl, aryl, or vinyl) bonded to the same oxygen atom, R–O–R′. Diethyl ether is used industrially as a solvent. Tetrahydrofuran (THF) is a solvent that is a cyclic ether. Thiols (R–S–H) and sulfides (R–S–R′) are sulfur (for oxygen) analogs of alcohols and ethers. ...
... An ether has two organic groups (alkyl, aryl, or vinyl) bonded to the same oxygen atom, R–O–R′. Diethyl ether is used industrially as a solvent. Tetrahydrofuran (THF) is a solvent that is a cyclic ether. Thiols (R–S–H) and sulfides (R–S–R′) are sulfur (for oxygen) analogs of alcohols and ethers. ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.