Aromatic Compounds
... Electrophilic Substitution The intermediate carbocation in electrophilic aromatic substitution is more stable than a typical alkyl carbocation because of resonance but much less stable than the starting benzene ring Comparison of alkene addition and aromatic substitution ...
... Electrophilic Substitution The intermediate carbocation in electrophilic aromatic substitution is more stable than a typical alkyl carbocation because of resonance but much less stable than the starting benzene ring Comparison of alkene addition and aromatic substitution ...
chemistry - Textbooks Online
... method - Calculations using densities and specific gravities - Calculation of formula weight - Understanding Avogadro’s number - Mole concept-mole fraction of the solvent and solute - Conversion of grams into moles and moles into grams Calculation of empirical formula from quantitative analysis and ...
... method - Calculations using densities and specific gravities - Calculation of formula weight - Understanding Avogadro’s number - Mole concept-mole fraction of the solvent and solute - Conversion of grams into moles and moles into grams Calculation of empirical formula from quantitative analysis and ...
CHAPTER 20 CARBOXYLIC ACIDS Organic Chemistry
... • Carboxylic acids exhibit dipole-dipole interactions because they have polar C—O and O—H bonds. • They also exhibit intermolecular hydrogen bonding. • Carboxylic acids often exist as dimers held together by two intermolecular hydrogen bonds. ...
... • Carboxylic acids exhibit dipole-dipole interactions because they have polar C—O and O—H bonds. • They also exhibit intermolecular hydrogen bonding. • Carboxylic acids often exist as dimers held together by two intermolecular hydrogen bonds. ...
Lecture 1: Key Concepts in Stereoselective Synthesis
... Overall, the reactions are exothermic but nearly ergoneutral. The electronic effect on this reaction is almost purely a result of kinetics, not thermodynamics. Factors, as the equilibrium constants, for these additions are similar (entries 2 vs. 1, 3 vs. 1). In contrast, steric properties of the ami ...
... Overall, the reactions are exothermic but nearly ergoneutral. The electronic effect on this reaction is almost purely a result of kinetics, not thermodynamics. Factors, as the equilibrium constants, for these additions are similar (entries 2 vs. 1, 3 vs. 1). In contrast, steric properties of the ami ...
haloalkanes and arenes
... manufacture was banned in the United States and many other countries in 1994. Uses of DDT DDT (p, p−dichlorodiphenyltrichloroethane) is one of the best known insecticides. It is very effective against mosquitoes and lice. But due its harmful effects, it was banned in the United States in 1973. Uses ...
... manufacture was banned in the United States and many other countries in 1994. Uses of DDT DDT (p, p−dichlorodiphenyltrichloroethane) is one of the best known insecticides. It is very effective against mosquitoes and lice. But due its harmful effects, it was banned in the United States in 1973. Uses ...
Chapter 19 Amines
... Weaker hydrogen bonds, so amines will have a lower boiling point than the corresponding alcohol. Tertiary amines cannot hydrogen-bond, so they have lower boiling points than primary and secondary amines. Chapter 19 ...
... Weaker hydrogen bonds, so amines will have a lower boiling point than the corresponding alcohol. Tertiary amines cannot hydrogen-bond, so they have lower boiling points than primary and secondary amines. Chapter 19 ...
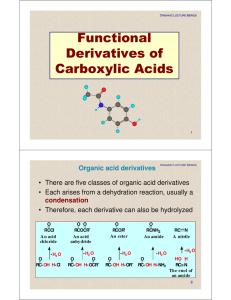
Functional Derivatives of Carboxylic Acids
... Reactions with Organolithium Organolithium compounds are even more powerful nucleophiles than Grignard reagents – they react with esters to give the same types of 2°and 3°alcohols as do Grignard reagents – and often in higher yields O RCOCH3 ...
... Reactions with Organolithium Organolithium compounds are even more powerful nucleophiles than Grignard reagents – they react with esters to give the same types of 2°and 3°alcohols as do Grignard reagents – and often in higher yields O RCOCH3 ...
esterification of palmitic acid with methanol in the
... CH3 (CH2)14COOH + CH3OH CH3 (CH2)14COOCH3 + H2O Palmitic acid ...
... CH3 (CH2)14COOH + CH3OH CH3 (CH2)14COOCH3 + H2O Palmitic acid ...
Chapter 14 Notes
... the lowest number. • Name and number other substituents as before. Examples: ...
... the lowest number. • Name and number other substituents as before. Examples: ...
Ethers - Home - KSU Faculty Member websites
... Know the different methods of naming ethers Know the physical properties of ethers Know the different methods used in preparation of ethers Know the reactions of opened ethers with HX Know the different methods used in synthesis of epoxides Know the reactions of epoxides with different nuc ...
... Know the different methods of naming ethers Know the physical properties of ethers Know the different methods used in preparation of ethers Know the reactions of opened ethers with HX Know the different methods used in synthesis of epoxides Know the reactions of epoxides with different nuc ...
First palladium- and nickel-catalyzed oxidative
... Early work from our laboratory employed preformed imidoosmium(VIII) reagents, which display unprecedented reactivity and selectivity toward selective diamination [3]. In addition, chiral versions of these reactions allowed for the development of diastereoselective [4] and enantioselective [5] alkene ...
... Early work from our laboratory employed preformed imidoosmium(VIII) reagents, which display unprecedented reactivity and selectivity toward selective diamination [3]. In addition, chiral versions of these reactions allowed for the development of diastereoselective [4] and enantioselective [5] alkene ...
Pincer Complexes. Applications in Catalysis
... impossible to be carried out with conventional diphosphine ligands. The versatility of these species to be modified and modulated both steric and electronically, makes these compounds an their corresponding transition metal derivatives attractive complexes to be continuously used in different challe ...
... impossible to be carried out with conventional diphosphine ligands. The versatility of these species to be modified and modulated both steric and electronically, makes these compounds an their corresponding transition metal derivatives attractive complexes to be continuously used in different challe ...
Ch. 11 Notes with Answers
... • The aldehyde form cannot itself get oxidized to carboxylic acid • Since PCC is used in absence of water, the aldehyde is not able to equilibrate with acetal and simply stays aldehyde. • Since it can’t convert to acetal, therefore no oxidation to carboxylic acid can occur 4. Chromic acid, by contra ...
... • The aldehyde form cannot itself get oxidized to carboxylic acid • Since PCC is used in absence of water, the aldehyde is not able to equilibrate with acetal and simply stays aldehyde. • Since it can’t convert to acetal, therefore no oxidation to carboxylic acid can occur 4. Chromic acid, by contra ...
molecules
... alkylhydroperoxides, hydrogen peroxide and periodates have been used these transformations [1]. In fact, the majority of the studies are directed toward understanding the mechanism of the catalytic activity of heme-containing enzymes such as cytochrome P-450. Homogeneous metalloporphyrin catalysts w ...
... alkylhydroperoxides, hydrogen peroxide and periodates have been used these transformations [1]. In fact, the majority of the studies are directed toward understanding the mechanism of the catalytic activity of heme-containing enzymes such as cytochrome P-450. Homogeneous metalloporphyrin catalysts w ...
Intermolecular bonding - Teacher instructions - Lesson element
... paves the way to an understanding of instantaneous dipole–induced dipole bonding and how this is present in all molecules, the strength increasing with the Mr. The relative strengths of the three types of intermolecular bond can be seen from the graph. This activity also offers an opportunity to rev ...
... paves the way to an understanding of instantaneous dipole–induced dipole bonding and how this is present in all molecules, the strength increasing with the Mr. The relative strengths of the three types of intermolecular bond can be seen from the graph. This activity also offers an opportunity to rev ...
No Slide Title
... This Powerpoint show is one of several produced to help students understand selected topics at AS and A2 level Chemistry. It is based on the requirements of the AQA and OCR specifications but is suitable for other examination boards. Individual students may use the material at home for revision purp ...
... This Powerpoint show is one of several produced to help students understand selected topics at AS and A2 level Chemistry. It is based on the requirements of the AQA and OCR specifications but is suitable for other examination boards. Individual students may use the material at home for revision purp ...
Study Guide Chapter 4 Alcohols and Alkyl Halides
... This compound has a five-carbon chain that bears a methyl substituent and a bromine. The numbering scheme that gives the lower number to the substituent closest to the end of the chain is chosen. Bromine is therefore at C-1, and methyl is a substituent at C-4. CH3CHCH2CH2CH2Br CH3 ...
... This compound has a five-carbon chain that bears a methyl substituent and a bromine. The numbering scheme that gives the lower number to the substituent closest to the end of the chain is chosen. Bromine is therefore at C-1, and methyl is a substituent at C-4. CH3CHCH2CH2CH2Br CH3 ...
Chapter 19 Amines
... aldehyde or ketone with hydroxylamine, then reduce the oxime. • To produce a 2 amine, react an aldehyde or ketone with a 1 amine, then reduce the imine. • To produce a 3 amine, react an aldehyde or ketone with a 2 amine, then reduce the imine salt. ...
... aldehyde or ketone with hydroxylamine, then reduce the oxime. • To produce a 2 amine, react an aldehyde or ketone with a 1 amine, then reduce the imine. • To produce a 3 amine, react an aldehyde or ketone with a 2 amine, then reduce the imine salt. ...
ch18-carboxylic acids
... l Reactions of Acyl Chlorides è Acyl chlorides are the most reactive acyl compounds and can be used to make any of the other derivatives è Since acyl chlorides are easily made from carboxylic acids they provide a way to synthesize any acyl compound from a carboxylic acid è Acyl chlorides react readi ...
... l Reactions of Acyl Chlorides è Acyl chlorides are the most reactive acyl compounds and can be used to make any of the other derivatives è Since acyl chlorides are easily made from carboxylic acids they provide a way to synthesize any acyl compound from a carboxylic acid è Acyl chlorides react readi ...
Document
... (IOB)23 have emerged as versatile oxidizing agents. Organoiodine(III) reagents in combination with other reagents have also shown interesting applications in organic synthesis. For example, Kita et al.24,25 have reported that iodosobenzene in combination with KBr (system i) in aqueous methanol can b ...
... (IOB)23 have emerged as versatile oxidizing agents. Organoiodine(III) reagents in combination with other reagents have also shown interesting applications in organic synthesis. For example, Kita et al.24,25 have reported that iodosobenzene in combination with KBr (system i) in aqueous methanol can b ...
Chpt 23Final7e
... of alanine (a) How do you account for the fact that the -NH3 + group of the conjugate acid of alanine is a stronger acid than the -NH3 + group of the conjugate acid of isopropylamine? The electron-withdrawing properties of the carboxyl group adjacent to the amine of alanine make the conjugate acid o ...
... of alanine (a) How do you account for the fact that the -NH3 + group of the conjugate acid of alanine is a stronger acid than the -NH3 + group of the conjugate acid of isopropylamine? The electron-withdrawing properties of the carboxyl group adjacent to the amine of alanine make the conjugate acid o ...
Microwave-Assisted Esterification of N -Acetyl-L-Phenylalanine Using Modified Mukaiyama s Reagents: A New Approach Involving Ionic Liquids
... CH3 1e X = EtSO4, 1f X = Tf2N [i.e. (CF3SO2)2N] Scheme 1. Structures of original Mukaiyama’s reagent (1a) and its derivatives (1b-1f). This paper was inspired by the resemblance of the original Mukaiyama’s reagent 1a with ionic liquids (ILs). As a brief background, ILs are ionic salts that are liqui ...
... CH3 1e X = EtSO4, 1f X = Tf2N [i.e. (CF3SO2)2N] Scheme 1. Structures of original Mukaiyama’s reagent (1a) and its derivatives (1b-1f). This paper was inspired by the resemblance of the original Mukaiyama’s reagent 1a with ionic liquids (ILs). As a brief background, ILs are ionic salts that are liqui ...
CHEM 494 Lecture 5 - UIC Department of Chemistry
... • fast step because small activation energy; positive and negative atoms bond fast • products are much lower in energy since they are neutral; exothermic reaction ...
... • fast step because small activation energy; positive and negative atoms bond fast • products are much lower in energy since they are neutral; exothermic reaction ...
Titania-catalysed oxidative dehydrogenation of ethyl lactate
... Due to the importance of pyruvic acid and its derivatives, these reactions were studied by several groups, using both gas and liquid phases. In gas-phase reactions, various solid catalysts were used, including TeO2 and MoO3.5–9 These processes give high yields of pyruvate, but they are energy-intens ...
... Due to the importance of pyruvic acid and its derivatives, these reactions were studied by several groups, using both gas and liquid phases. In gas-phase reactions, various solid catalysts were used, including TeO2 and MoO3.5–9 These processes give high yields of pyruvate, but they are energy-intens ...
4 ORGANIC CHEMISTRY: STRUCTURE AND NOMENCLATURE
... O1.4 ROTATION AROUND COC BONDS It is easy to fall into the trap of thinking about the ethane molecule as if it were static. Nothing could be further from the truth. At room temperature, the average velocity of an ethane molecule is about 500 m/s—more than twice the speed of a Boeing 747. While it mo ...
... O1.4 ROTATION AROUND COC BONDS It is easy to fall into the trap of thinking about the ethane molecule as if it were static. Nothing could be further from the truth. At room temperature, the average velocity of an ethane molecule is about 500 m/s—more than twice the speed of a Boeing 747. While it mo ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.