Aromatic compounds
... electrophile attaches to a C in ring • In step 2, resonance energy is regained with loss of proton (H+) • Step 1 is slow since it requires so much energy (Ea), thus is ratedetermining step • Step 2 is fast with low Ea ...
... electrophile attaches to a C in ring • In step 2, resonance energy is regained with loss of proton (H+) • Step 1 is slow since it requires so much energy (Ea), thus is ratedetermining step • Step 2 is fast with low Ea ...
Organic Chemistry II with Dr Roche
... Since ethers are relatively unreactive and are strongly polar (due to the lone pairs on the oxygen), they are commonly used as solvents for organic reactions. (Diethyl ether and THF, the Grignard reaction). Ethers will often form complexes with molecules that have vacant orbitals, enabling ‘unstable ...
... Since ethers are relatively unreactive and are strongly polar (due to the lone pairs on the oxygen), they are commonly used as solvents for organic reactions. (Diethyl ether and THF, the Grignard reaction). Ethers will often form complexes with molecules that have vacant orbitals, enabling ‘unstable ...
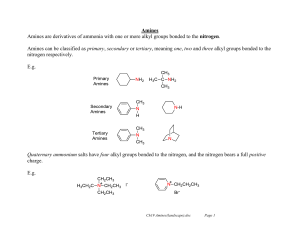
Amines Amines are derivatives of ammonia with one or more alkyl
... To achieve a conformation suitable for E2 to occur, C-3 must rotate and place a Hydrogen anti to the bulky leaving group. To remove the proton from C-1 ( Hofmann product), any of the three staggered conformations allow the E2 mechanism to operate. The Hofmann product dominates since elimination of ...
... To achieve a conformation suitable for E2 to occur, C-3 must rotate and place a Hydrogen anti to the bulky leaving group. To remove the proton from C-1 ( Hofmann product), any of the three staggered conformations allow the E2 mechanism to operate. The Hofmann product dominates since elimination of ...
Molecular Orbitals and Hybridisation
... a familiar tetrahedral shape, with a maximum possible angle between each orbital of 109.5°. ...
... a familiar tetrahedral shape, with a maximum possible angle between each orbital of 109.5°. ...
Advanced Practical Organic Chemistry
... The simplest hydrocarbon is methane, CH4. This is the simplest member of a series of hydrocarbons. Each successive member of the series has one more carbon atom than the preceding member. This is shown in the table below: ...
... The simplest hydrocarbon is methane, CH4. This is the simplest member of a series of hydrocarbons. Each successive member of the series has one more carbon atom than the preceding member. This is shown in the table below: ...
10.1 Intro to Organic Chemistry 10.1 Organic Chemistry
... 10.1.7 Deduce structural formulas for the isomers of the straightchain alkenes up to C6. 10.1.8 Apply IUPAC rules for naming the isomers of the straight-chain alkenes up to C6. 10.1.9 Deduce structural formulas for compounds containing up to six carbon atoms with one of the following functional grou ...
... 10.1.7 Deduce structural formulas for the isomers of the straightchain alkenes up to C6. 10.1.8 Apply IUPAC rules for naming the isomers of the straight-chain alkenes up to C6. 10.1.9 Deduce structural formulas for compounds containing up to six carbon atoms with one of the following functional grou ...
Organic Chemistry
... our planet glides majestically in space. Under these ideal conditions, the presence of liquid water as well as the formation and stable existence of chemical bonds that compose living systems are possible. These living systems grow and reproduce on Earth, move gracefully in the oceans, and float eff ...
... our planet glides majestically in space. Under these ideal conditions, the presence of liquid water as well as the formation and stable existence of chemical bonds that compose living systems are possible. These living systems grow and reproduce on Earth, move gracefully in the oceans, and float eff ...
Aromatic heterocycles 1: structures and reactions
... probably about two-thirds of—organic compounds belong to this class, and they number among them some of the most significant compounds for human beings. If we think only of drugs we can define the history of medicine by heterocycles. Even in the sixteenth century quinine was used to prevent and trea ...
... probably about two-thirds of—organic compounds belong to this class, and they number among them some of the most significant compounds for human beings. If we think only of drugs we can define the history of medicine by heterocycles. Even in the sixteenth century quinine was used to prevent and trea ...
synthesis, chemistry and optical resol
... hexamethylphosphorous triamide1° to give the trans adduct 11 in 72% yield after purification (Scheme I). A minor amount of the corresponding 5,9& product was also obtained (-4% yield). Reduction of the trans product 11 with L-Selectride in T H F at -78 “ C produced an -2.7:l mixture of the C-7 a- an ...
... hexamethylphosphorous triamide1° to give the trans adduct 11 in 72% yield after purification (Scheme I). A minor amount of the corresponding 5,9& product was also obtained (-4% yield). Reduction of the trans product 11 with L-Selectride in T H F at -78 “ C produced an -2.7:l mixture of the C-7 a- an ...
Lecture - Ch 17
... Worked Example • When the 1H NMR spectrum of an alcohol is run in dimethyl sulfoxide solvent rather than in chloroform, exchange of the O–H proton is slow and spin–spin splitting is seen between the O–H proton and C–H protons on the adjacent carbon What spin multiplicities are expected for the hydr ...
... Worked Example • When the 1H NMR spectrum of an alcohol is run in dimethyl sulfoxide solvent rather than in chloroform, exchange of the O–H proton is slow and spin–spin splitting is seen between the O–H proton and C–H protons on the adjacent carbon What spin multiplicities are expected for the hydr ...
Sodium tungstate dihydrate - Revista Virtual de Química
... The oxidation of organosulfur compounds is the most straightforward method for the preparation of sulfoxides and sulfones, important as commodity chemicals or pharmaceuticals. Noyori and co-workers reported an oxidative process using sodium tungstate dihydrate as catalyst, 30% aqueous H2O2 as oxidan ...
... The oxidation of organosulfur compounds is the most straightforward method for the preparation of sulfoxides and sulfones, important as commodity chemicals or pharmaceuticals. Noyori and co-workers reported an oxidative process using sodium tungstate dihydrate as catalyst, 30% aqueous H2O2 as oxidan ...
1 THE BARTON-McCOMBIE REACTION STUART W. McCOMBIE 28
... to Professor Samir Zard for valuable discussions. This chapter is dedicated to the memory of Professor Derek H. R. Barton, mentor and friend. ...
... to Professor Samir Zard for valuable discussions. This chapter is dedicated to the memory of Professor Derek H. R. Barton, mentor and friend. ...
Metal Carbenes
... The late transition metals, being more electronegative, have stabilized M(dp) orbitals resulting in weak M→C π back donation. ...
... The late transition metals, being more electronegative, have stabilized M(dp) orbitals resulting in weak M→C π back donation. ...
Carbonyl Compounds I. Aldehydes and Ketones
... oxidized carbonyl compounds such as aldehydes and ketones are not so widespread in nature. That is not to say that they are unimportant. To the contrary. Aldehydes and ketones are of great importance both in biological chemistry and in synthetic organic chemistry. However, the high reactivity of the ...
... oxidized carbonyl compounds such as aldehydes and ketones are not so widespread in nature. That is not to say that they are unimportant. To the contrary. Aldehydes and ketones are of great importance both in biological chemistry and in synthetic organic chemistry. However, the high reactivity of the ...
Question number - Bethlehem College .::. Welcome
... Heating under reflux increases the rate of reaction and the condenser returns evaporated organic molecules to the reaction flask by condensing the vapour that is produced on heating. (iii) Sulfuric acid = catalyst (or dehydrating agent ) Anhydrous sodium sulfate = To remove last traces of water from ...
... Heating under reflux increases the rate of reaction and the condenser returns evaporated organic molecules to the reaction flask by condensing the vapour that is produced on heating. (iii) Sulfuric acid = catalyst (or dehydrating agent ) Anhydrous sodium sulfate = To remove last traces of water from ...
Microsoft Word
... to the presence of reaction species in the following order. s(pure methanol) > s[onitrophenol(0.36 mol.dm-3 ) in methanol] > s[o-a minopheno 1(0.36 mol.dm-3 ) in methanol]. However, the addition of water to the methanol-OAP and methanol-ONP-OAP systems causes an increase in the solubility. Neverthe ...
... to the presence of reaction species in the following order. s(pure methanol) > s[onitrophenol(0.36 mol.dm-3 ) in methanol] > s[o-a minopheno 1(0.36 mol.dm-3 ) in methanol]. However, the addition of water to the methanol-OAP and methanol-ONP-OAP systems causes an increase in the solubility. Neverthe ...
+ :O
... 1) Carboxylic acids are readily identified by a C=O stretch near 1715 cm-1 and a broad O-H stretch between 2400-3500 cm-1. 2) Esters show the C=O stretch somewhere between 1735-1750 cm-1 and C-O stretch at 1000-1300 cm-1. 3) Acyl chlorides have their C=O band at 1785-1815 cm-1. 4) Anhydrides have tw ...
... 1) Carboxylic acids are readily identified by a C=O stretch near 1715 cm-1 and a broad O-H stretch between 2400-3500 cm-1. 2) Esters show the C=O stretch somewhere between 1735-1750 cm-1 and C-O stretch at 1000-1300 cm-1. 3) Acyl chlorides have their C=O band at 1785-1815 cm-1. 4) Anhydrides have tw ...
PHYSICOCHEMICAL PROPERTIES OF ORGANIC MEDICINAL
... CH3(CH2)4-OH (1-Pentanol) As a result of intermolecular H-bonding, alcohols have significantly higher boiling points than corresponding simple hydrocarbons. For example, simple hydrocarbons with fewer than five ...
... CH3(CH2)4-OH (1-Pentanol) As a result of intermolecular H-bonding, alcohols have significantly higher boiling points than corresponding simple hydrocarbons. For example, simple hydrocarbons with fewer than five ...
عرض تقديمي من PowerPoint
... Heterocyclic analogues of Cyclopropane All three membered rings have one major property in common- a strained ring which confers great reactivity on the compounds in comparison with their openchain analogues. ...
... Heterocyclic analogues of Cyclopropane All three membered rings have one major property in common- a strained ring which confers great reactivity on the compounds in comparison with their openchain analogues. ...
Chapter 26 Review
... compounds will produce the most energy when completely oxidized: a) butane, or b) butanol Look up bond energy values and work out the correct answer What is a test for alcohols? What is the expected product when 1-propanol is oxidized? ...
... compounds will produce the most energy when completely oxidized: a) butane, or b) butanol Look up bond energy values and work out the correct answer What is a test for alcohols? What is the expected product when 1-propanol is oxidized? ...
PROFESSOR SIR DEREK H. R. BARTON AND HETEROCYCLES
... In a conceptually similar approach, he developed an efficient radical deamination method based on the reaction of isocyanides with tributylstannane. The corresponding reduction of lsothiocyanldes and of isoselenocyanates also affords the deaminated products. Since the order of reactivity is tertiary ...
... In a conceptually similar approach, he developed an efficient radical deamination method based on the reaction of isocyanides with tributylstannane. The corresponding reduction of lsothiocyanldes and of isoselenocyanates also affords the deaminated products. Since the order of reactivity is tertiary ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.