Preparation of a haloalkane
... The stopper must be removed before the liquid will flow. It’s safer to drain the liquid into a beaker – that way if you make a mistake you can pour it back! ...
... The stopper must be removed before the liquid will flow. It’s safer to drain the liquid into a beaker – that way if you make a mistake you can pour it back! ...
Glossary of Key Terms in Chapter Two
... gain of oxygen or loss of hydrogen; e.g., the conversion of an alcohol to an aldehyde or ketone via the use of an oxidizing agent. phenol (12.7) an organic compound that contains a hydroxyl group (-OH) attached to a benzene ring. primary (1˚) alcohol (12.4) an alcohol with the general formula RCH2OH ...
... gain of oxygen or loss of hydrogen; e.g., the conversion of an alcohol to an aldehyde or ketone via the use of an oxidizing agent. phenol (12.7) an organic compound that contains a hydroxyl group (-OH) attached to a benzene ring. primary (1˚) alcohol (12.4) an alcohol with the general formula RCH2OH ...
Chapter 8 - Alkenes and Alkynes II
... 8.7 - Alcohols from Alkenes through Hydroboration-Oxidation: Anti-Markovnikov Syn Hydration - The use of B2 H6 or a solution of BH3 :THF can cause Anti-Markovnikov hydration of a double bond - The addition of a boron atom and a hydrogen atom to a double bond is called hydroboration - Hydroboration- ...
... 8.7 - Alcohols from Alkenes through Hydroboration-Oxidation: Anti-Markovnikov Syn Hydration - The use of B2 H6 or a solution of BH3 :THF can cause Anti-Markovnikov hydration of a double bond - The addition of a boron atom and a hydrogen atom to a double bond is called hydroboration - Hydroboration- ...
Haloalkanes and Haloarenes
... 10.4.1 From Alcohols Alkyl halides are best prepared from alcohols, which are easily accessible. The hydroxyl group of an alcohol is replaced by halogen on reaction with concentrated halogen acids, phosphorus halides or thionyl chloride. Thionyl chloride is preferred because the other two products a ...
... 10.4.1 From Alcohols Alkyl halides are best prepared from alcohols, which are easily accessible. The hydroxyl group of an alcohol is replaced by halogen on reaction with concentrated halogen acids, phosphorus halides or thionyl chloride. Thionyl chloride is preferred because the other two products a ...
Addition Reactions
... acid-catalyzed hydration of an alkene is regioselective; hydrogen adds preferentially to the sp2 carbon with less # of hydrogens. ...
... acid-catalyzed hydration of an alkene is regioselective; hydrogen adds preferentially to the sp2 carbon with less # of hydrogens. ...
World of Chemistry Chapter 20—Organic Chemistry
... release energy that originally came from the sun…photosynthesis stored energy in the plants and by burning their decay products we can release that energy. These are fossil fuels Section 20.6—Reactions of Alkanes ...
... release energy that originally came from the sun…photosynthesis stored energy in the plants and by burning their decay products we can release that energy. These are fossil fuels Section 20.6—Reactions of Alkanes ...
Summary from Organic Chemistry Packet:
... – Alkynes: carbon-carbon triple bonds as well as single bonds. – Aromatic: hydrocarbons containing benzene rings. ...
... – Alkynes: carbon-carbon triple bonds as well as single bonds. – Aromatic: hydrocarbons containing benzene rings. ...
COURSE: Organic chemistry ACADEMIC YEAR:2016/2017 TYPE
... student to understand the physical properties and the chemical behavior of every organic compounds. PRE-REQUIREMENTS General and inorganic chemistry SYLLABUS Electronic configuration. Bonds. Representing molecules. Hydtocarbons: alkanes, alkenes, arenes, alkynes. Compounds containing nitrogen: sp3 n ...
... student to understand the physical properties and the chemical behavior of every organic compounds. PRE-REQUIREMENTS General and inorganic chemistry SYLLABUS Electronic configuration. Bonds. Representing molecules. Hydtocarbons: alkanes, alkenes, arenes, alkynes. Compounds containing nitrogen: sp3 n ...
Slide 1
... Dependence of the rate of the reaction of acetone with hydroxylamine on the pH of the reaction: a pH rate profile ...
... Dependence of the rate of the reaction of acetone with hydroxylamine on the pH of the reaction: a pH rate profile ...
Organic Chemistry
... (i) 1-Bromobutane (ii) 2-Bromobutane. Ans2. 2-Bromobutane contain one chiral C-atom. So, it is optically active, ...
... (i) 1-Bromobutane (ii) 2-Bromobutane. Ans2. 2-Bromobutane contain one chiral C-atom. So, it is optically active, ...
Organic Tutorial 1st Year MT03
... Peter Sykes,“A Guidebook to Mechanism in Organic Chemistry”, and Eames & Peach “Stereochemistry at a Glance”. Notes and Questions a) Summary on not more than 6 sides. This should outline the possible mechanisms and the evidence on which they are based, in particular the evidence for inversion during ...
... Peter Sykes,“A Guidebook to Mechanism in Organic Chemistry”, and Eames & Peach “Stereochemistry at a Glance”. Notes and Questions a) Summary on not more than 6 sides. This should outline the possible mechanisms and the evidence on which they are based, in particular the evidence for inversion during ...
Organic Chemistry
... • The weak attractive forces between the molecules have to be broken if the hydrocarbon is to boil • The longer the hydrocarbon molecule is, the stronger the intermolecular forces are • The shorter chains are more volatile – they form a vapour ...
... • The weak attractive forces between the molecules have to be broken if the hydrocarbon is to boil • The longer the hydrocarbon molecule is, the stronger the intermolecular forces are • The shorter chains are more volatile – they form a vapour ...
Chapter 10: Alkyl Halides
... Allylic Bromination with NBS is analogous to the radical reaction with an alkane, a halogen and uv light (Ch. 5). The NBS can be thought of as producing a Br radical. The Br radical removes a hydrogen, leaving an allylic radical and forming HBr. This allylic radical reacts with Br2 (which is formed ...
... Allylic Bromination with NBS is analogous to the radical reaction with an alkane, a halogen and uv light (Ch. 5). The NBS can be thought of as producing a Br radical. The Br radical removes a hydrogen, leaving an allylic radical and forming HBr. This allylic radical reacts with Br2 (which is formed ...
Glossary of Key Terms in Chapter Two
... Glossary of Key Terms in Chapter Eleven addition polymer (11.5) a polymer prepared by sequential addition of monomers. addition reaction (11.5) a reaction in which two molecules add together to form a new molecule; often involves the addition of one molecule to a double or triple bond in an unsatura ...
... Glossary of Key Terms in Chapter Eleven addition polymer (11.5) a polymer prepared by sequential addition of monomers. addition reaction (11.5) a reaction in which two molecules add together to form a new molecule; often involves the addition of one molecule to a double or triple bond in an unsatura ...
ADDITION REACTIONS
... • Polymers,can be made artificially and these are usually referred to as plastics, but there are also a great number of naturally occurring polymers. • One type of polymerisation reaction is known as addition polymerisation. In this the monomers contain double bonds and in the addition reaction new ...
... • Polymers,can be made artificially and these are usually referred to as plastics, but there are also a great number of naturally occurring polymers. • One type of polymerisation reaction is known as addition polymerisation. In this the monomers contain double bonds and in the addition reaction new ...
Alkene Addition Reactions
... The rate-‐determining step of the reaction is the formation of a carbocation, which represents the most stable on the immediate double bond. The order of carbocation stability is 3o > 2o > 1o. ...
... The rate-‐determining step of the reaction is the formation of a carbocation, which represents the most stable on the immediate double bond. The order of carbocation stability is 3o > 2o > 1o. ...
Exam - Chemistry With BT
... More than one step may be required. Show all reagents and intermediate products. ...
... More than one step may be required. Show all reagents and intermediate products. ...
Chapter 7
... Rearrangements • Only the carbocation rearranges, so dehydration of primary alcohols can not have rearrangements since they are E2 and not carbocation is formed! • However, as we will see in Ch 8, the alkene product can react with the acid by using its pi electrons to abstract a proton from an acid ...
... Rearrangements • Only the carbocation rearranges, so dehydration of primary alcohols can not have rearrangements since they are E2 and not carbocation is formed! • However, as we will see in Ch 8, the alkene product can react with the acid by using its pi electrons to abstract a proton from an acid ...
AMINO ACIDS Ethan Secor, John N. Gitua (Mentor)
... METALLORGANIC SYNTHESIS OF -AMINO ACIDS Ethan Secor, John N. Gitua (Mentor) Department of Chemistry, College of Arts and Sciences Drake University RESULTS ...
... METALLORGANIC SYNTHESIS OF -AMINO ACIDS Ethan Secor, John N. Gitua (Mentor) Department of Chemistry, College of Arts and Sciences Drake University RESULTS ...
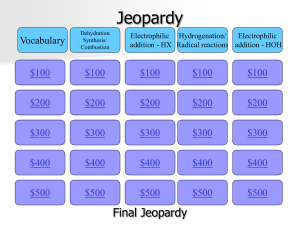
Jeopardy
... What is the name of the rule that states that the nucleophilic part of the reagent will bind with the most stable carbon of the substrate? ...
... What is the name of the rule that states that the nucleophilic part of the reagent will bind with the most stable carbon of the substrate? ...
Chapter 7
... Rearrangements • Only the carbocation rearranges, so dehydration of primary alcohols can not have rearrangements since they are E2 and not carbocation is formed! • However, as we will see in Ch 8, the alkene product can react with the acid by using its pi electrons to abstract a proton from an acid ...
... Rearrangements • Only the carbocation rearranges, so dehydration of primary alcohols can not have rearrangements since they are E2 and not carbocation is formed! • However, as we will see in Ch 8, the alkene product can react with the acid by using its pi electrons to abstract a proton from an acid ...
CHEM 203 Topics Discussed on Nov. 20 Principle: protonation of
... Electrophilic character of the above reagents and facile reaction thereof with nucleophiles Principle: the above reagents rely on the nucleophilic properties of the OH group to achieve conversion of alcohols into alkyl halides Principle: only primary and secondary alcohols are sufficiently nucleophi ...
... Electrophilic character of the above reagents and facile reaction thereof with nucleophiles Principle: the above reagents rely on the nucleophilic properties of the OH group to achieve conversion of alcohols into alkyl halides Principle: only primary and secondary alcohols are sufficiently nucleophi ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.