TYPES OF ORGANIC CHEMICAL REACTIONS
... A substitution reaction occurs whenever one atom of an organic molecule is replaced by another. Substitution reactions often require heat and/or a catalyst in order to occur. Example: Substitution of an alkane H H H C H + Cl2 H C Cl + HCl H H HEAT ...
... A substitution reaction occurs whenever one atom of an organic molecule is replaced by another. Substitution reactions often require heat and/or a catalyst in order to occur. Example: Substitution of an alkane H H H C H + Cl2 H C Cl + HCl H H HEAT ...
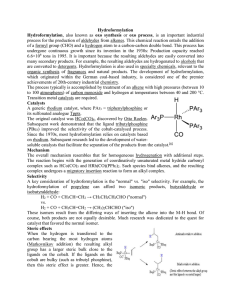
Hydroformylation Hydroformylation, also known as oxo synthesis or
... Hydroformylation Hydroformylation, also known as oxo synthesis or oxo process, is an important industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has und ...
... Hydroformylation Hydroformylation, also known as oxo synthesis or oxo process, is an important industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has und ...
SCH4U Organic Chemistry Portfolio Name: This portfolio is due on
... 5. Nomex is a polymer used to make flame-resistant clothing for firefighters. A portion of its structure is provided below. Write a polymerization reaction showing its production from monomers. What type of reaction is this? ...
... 5. Nomex is a polymer used to make flame-resistant clothing for firefighters. A portion of its structure is provided below. Write a polymerization reaction showing its production from monomers. What type of reaction is this? ...
2d Oxidation of Food Homework
... a) Using information from the table, describe two ways in which differences in the structures affect boiling point of isomeric alcohols. b) Predict a boiling point for hexan-2-ol. ...
... a) Using information from the table, describe two ways in which differences in the structures affect boiling point of isomeric alcohols. b) Predict a boiling point for hexan-2-ol. ...
Nucleophilic substitution at saturated carbon
... Nucleophilic attack with inversion gives the trans product. As the epoxide is up, attack has to come from underneath. Notice that the new C–N bond is down and that the H atom at the site of attack was down in the epoxide but is up in the ...
... Nucleophilic attack with inversion gives the trans product. As the epoxide is up, attack has to come from underneath. Notice that the new C–N bond is down and that the H atom at the site of attack was down in the epoxide but is up in the ...
Chemistry 123: Physical and Organic Chemistry
... 13) For graphite which would be numerically larger? (A) ΔfG° (B) ΔfH° (C) ΔS° 14) Which of the following would have the greater ΔS? (A) C(s) (B) C(g) (C) CO(g) (D) CO2(g) 15) If a reaction has a half-life of one day and it is initiated on Monday, how much ...
... 13) For graphite which would be numerically larger? (A) ΔfG° (B) ΔfH° (C) ΔS° 14) Which of the following would have the greater ΔS? (A) C(s) (B) C(g) (C) CO(g) (D) CO2(g) 15) If a reaction has a half-life of one day and it is initiated on Monday, how much ...
Chapter 7 Alkenes and Alkynes I
... The relative stabilities of alkenes can be measured using the exothermic heats of hydrogenation ...
... The relative stabilities of alkenes can be measured using the exothermic heats of hydrogenation ...
TT T p
... functional'groups have been added and the carbon atom in the straight chain to'which they are attached.A few gxamplesof halogen derivatives are Shown.in Figure 9-8 on page 74. Note that structures(b) and (c) are isomers. ...
... functional'groups have been added and the carbon atom in the straight chain to'which they are attached.A few gxamplesof halogen derivatives are Shown.in Figure 9-8 on page 74. Note that structures(b) and (c) are isomers. ...
Organic Chemistry I: Reactions and Overview
... 5.1 Naming Enantiomers via the -R and -S System 1. Each of the four groups attached to the chirality center is assigned a priority of 1, 2, 3, or 4. Priority is assigned on the basis of the atomic number of the atom that is directly attached to the chirality center. The group with the highest atomic ...
... 5.1 Naming Enantiomers via the -R and -S System 1. Each of the four groups attached to the chirality center is assigned a priority of 1, 2, 3, or 4. Priority is assigned on the basis of the atomic number of the atom that is directly attached to the chirality center. The group with the highest atomic ...
Halogenoalkanes
... Note that this reaction is very exothermic so the solution must be cold, or dry ice (solid CO2 at –78o C used instead). ...
... Note that this reaction is very exothermic so the solution must be cold, or dry ice (solid CO2 at –78o C used instead). ...
Exam 4
... 3) The sum of the masses of two hydrogen atoms (mass number 1) and two neutrons is 4.0330. Why does this differ from the mass of a helium atom (4.0026)? a) Some hydrogen atoms are heavier than others b) The difference is the binding energy of the helium nucleus c) The difference is the experimental ...
... 3) The sum of the masses of two hydrogen atoms (mass number 1) and two neutrons is 4.0330. Why does this differ from the mass of a helium atom (4.0026)? a) Some hydrogen atoms are heavier than others b) The difference is the binding energy of the helium nucleus c) The difference is the experimental ...
CH 2
... C≣C bond results from the overlap of two sphybridized carbon atoms and consists of one spsp s bond and two p-p p bonds The general formula is CnH2n-2 Alkynes are named by general rules similar to those used for alkanes and alkenes The suffix –yne Internal alkynes and terminal alkynes ...
... C≣C bond results from the overlap of two sphybridized carbon atoms and consists of one spsp s bond and two p-p p bonds The general formula is CnH2n-2 Alkynes are named by general rules similar to those used for alkanes and alkenes The suffix –yne Internal alkynes and terminal alkynes ...
-23- ORGANIC CHEMISTRY A. STRUCTURE AND ISOMERISM 1
... (a) reaction with acid (acid-base) - e.g. HCl, CH3COOH, etc. (b) amide formation (substitution) - carboxylic acids/heat Phenols (a) reaction with strong base (acid-base) - NaOH (b) ester formation (substitution) - anhydrides/H+ ...
... (a) reaction with acid (acid-base) - e.g. HCl, CH3COOH, etc. (b) amide formation (substitution) - carboxylic acids/heat Phenols (a) reaction with strong base (acid-base) - NaOH (b) ester formation (substitution) - anhydrides/H+ ...
Introduction (HL)
... If both enantiomers are equally present, it is called a racemic mixture or racemate. The two enantiomers rotate the plane of the polarized light by the same amount but in opposite directions. The rotations cancel each other out and the mixture appears to be optically inactive. ...
... If both enantiomers are equally present, it is called a racemic mixture or racemate. The two enantiomers rotate the plane of the polarized light by the same amount but in opposite directions. The rotations cancel each other out and the mixture appears to be optically inactive. ...
Workshop 5
... same reaction at a lower temperature. The Pb-C bond energy in (CH3)4Pb is 49 kcal/mol. a. Show the initiation and propagation steps for the chlorination of CH4 using (CH3)4Pb with CH4 and Cl2. Explain why lower temperatures are needed for the halogenation reaction using (CH3)4Pb as the initiator tha ...
... same reaction at a lower temperature. The Pb-C bond energy in (CH3)4Pb is 49 kcal/mol. a. Show the initiation and propagation steps for the chlorination of CH4 using (CH3)4Pb with CH4 and Cl2. Explain why lower temperatures are needed for the halogenation reaction using (CH3)4Pb as the initiator tha ...
AP Chemistry
... Addition reactions are a good test for a double bond. A diatomic bromine solution of carbon tetrachloride is reacted with the suspected compound. If a double bond is present, red color (from Br2) will quickly fade. ...
... Addition reactions are a good test for a double bond. A diatomic bromine solution of carbon tetrachloride is reacted with the suspected compound. If a double bond is present, red color (from Br2) will quickly fade. ...
Organic Compounds
... hydrocarbon derivatives have one or more hydrogen atoms replaced by another nonmetallic atom eg. bromomethane - CH3Br methanol - CH3OH ...
... hydrocarbon derivatives have one or more hydrogen atoms replaced by another nonmetallic atom eg. bromomethane - CH3Br methanol - CH3OH ...
11. Reactions of Alkyl Halides
... species and the rate constant of the step • The highest energy transition state point on the diagram is that for the rate determining step (which is not always the highest barrier) • This is the not the greatest difference but the absolute highest point (Figures 11.8 – the same step is rate-determin ...
... species and the rate constant of the step • The highest energy transition state point on the diagram is that for the rate determining step (which is not always the highest barrier) • This is the not the greatest difference but the absolute highest point (Figures 11.8 – the same step is rate-determin ...
Document
... • Atoms other than hydrogen or carbon covalently bonded to a carbon atom in an organic molecule. • Most commonly oxygen, nitrogen, or the halogens. • The presence of a functional group drastically changes the chemical properties of a molecule. ...
... • Atoms other than hydrogen or carbon covalently bonded to a carbon atom in an organic molecule. • Most commonly oxygen, nitrogen, or the halogens. • The presence of a functional group drastically changes the chemical properties of a molecule. ...
Q1. Give I.U.P.A..C Name of the following Organic Compound. 1 CH
... (1) ICl is more reactive than I2. (2) Why does NO2 dimerise? (3) H2S is less acidic than H2 Te why? OR (1) Which form of sulphur shows paramagnetic behaviour? (2) Halogens have maximum negative electron gain Enthalpy in the respective periods of the ...
... (1) ICl is more reactive than I2. (2) Why does NO2 dimerise? (3) H2S is less acidic than H2 Te why? OR (1) Which form of sulphur shows paramagnetic behaviour? (2) Halogens have maximum negative electron gain Enthalpy in the respective periods of the ...
Organic and Biochemical Compounds
... • Alkenes- are hydrocarbons that have only single covalent bonds • Methane- CH4 has only C-H bonds • Ethane- C2H6 has a C-C bond in addition to six C-H bonds • Propane-C3H8 has 3 bonded carbon atoms, each carbon forms three bonds with three hydrogen atoms ...
... • Alkenes- are hydrocarbons that have only single covalent bonds • Methane- CH4 has only C-H bonds • Ethane- C2H6 has a C-C bond in addition to six C-H bonds • Propane-C3H8 has 3 bonded carbon atoms, each carbon forms three bonds with three hydrogen atoms ...
Hydrocarbons - OurTeachersPage.com
... •Each functional group gives the molecule distinctive chemical & physical properties. •Molecules with functional groups contain at least one atom that is not C or H. Not hydrocarbons! ...
... •Each functional group gives the molecule distinctive chemical & physical properties. •Molecules with functional groups contain at least one atom that is not C or H. Not hydrocarbons! ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.