Organic Chemistry The chemistry of carbon compounds. Carbon
... This test used to distinguish between alcohols according to rate of reaction 30> 20> 10> methanol Tertiary alcohol reacts directly with Lucas reagent. 3- Oxidation by cold KMnO4 KMnO4/ H2O ...
... This test used to distinguish between alcohols according to rate of reaction 30> 20> 10> methanol Tertiary alcohol reacts directly with Lucas reagent. 3- Oxidation by cold KMnO4 KMnO4/ H2O ...
Biology 2B-1 - secondary
... – Organic compounds are based on carbon and its unusual bonding characteristics, They all contain carbon, most organic compounds under natural circumstances are the product of biosynthesis – Inorganic substances are derived from nonliving material and lack carbon ...
... – Organic compounds are based on carbon and its unusual bonding characteristics, They all contain carbon, most organic compounds under natural circumstances are the product of biosynthesis – Inorganic substances are derived from nonliving material and lack carbon ...
SNC2DExamChemistryreview
... 19. Predict if a reaction will happen using an activity series for the equation below. c) Li+HCl b) F2+MgCl2-> 20. The reaction of magnesium and oxygen produces a bright white light and heat. Mg(s) + O2 (g) 2MgO(g) a) What type of reaction is this? b) What are the reactants? What are the products ...
... 19. Predict if a reaction will happen using an activity series for the equation below. c) Li+HCl b) F2+MgCl2-> 20. The reaction of magnesium and oxygen produces a bright white light and heat. Mg(s) + O2 (g) 2MgO(g) a) What type of reaction is this? b) What are the reactants? What are the products ...
2009_outline_4
... Do Not turn in, answers available in "Study Guide and Solutions Manual for Organic Chemistry" for Solomons. This is available in the Bookstore or can be borrowed from Cameron Library's Reserve Reading Room ...
... Do Not turn in, answers available in "Study Guide and Solutions Manual for Organic Chemistry" for Solomons. This is available in the Bookstore or can be borrowed from Cameron Library's Reserve Reading Room ...
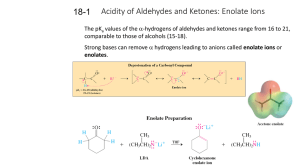
18-1 Enolates (PPT)
... For ordinary aldehydes and ketones, only traces of the enol form are present. The enol form is less stable by 8-12 kcal mol-1. However, for acetaldehyde, the enol form is about 100-times more stable than that of acetone because the less substituted aldehyde carbonyl is more stable than the more subs ...
... For ordinary aldehydes and ketones, only traces of the enol form are present. The enol form is less stable by 8-12 kcal mol-1. However, for acetaldehyde, the enol form is about 100-times more stable than that of acetone because the less substituted aldehyde carbonyl is more stable than the more subs ...
syllabus for entrance examination - NTU.edu
... ELECTRONIC EFFECTS AND REACTION TYPES The nature of the ...
... ELECTRONIC EFFECTS AND REACTION TYPES The nature of the ...
PPT: Intro to Organic Chemistry
... H H H H H –C–C–H + Cl2 H–C–C–Cl + HCl H H H H If more chlorine is provided, the reaction will produce... H H H H H –C–C–Cl + Cl2 Cl–C–C–Cl + HCl H H H H ...
... H H H H H –C–C–H + Cl2 H–C–C–Cl + HCl H H H H If more chlorine is provided, the reaction will produce... H H H H H –C–C–Cl + Cl2 Cl–C–C–Cl + HCl H H H H ...
CHMY_271_practice_exam_3
... 11. (6 pt) If the following alkyl halide were to undergo elimination, predict the major product in each case, and explain your answer. You do not need to draw out the mechanism, but knowing the mechanism will help you to predict reasonable products. Br ...
... 11. (6 pt) If the following alkyl halide were to undergo elimination, predict the major product in each case, and explain your answer. You do not need to draw out the mechanism, but knowing the mechanism will help you to predict reasonable products. Br ...
CH 6
... • Regiospecific – one product forms where two are possible • If both ends have similar substitution, then not regiospecific ...
... • Regiospecific – one product forms where two are possible • If both ends have similar substitution, then not regiospecific ...
ORGANIC CHEMISTRY 03 JULY 2014 Lesson Description
... Fully explain the trend in QUESTION 2.2.1. ...
... Fully explain the trend in QUESTION 2.2.1. ...
Organic Chemistry
... Alkanes burn exothermically to produce carbon dioxide and water if there is a plentiful supply of oxygen. This is known as complete combustion. e.g. CH4 + 2O2 CO2 + 2H2O Write equations for the complete combustion of ...
... Alkanes burn exothermically to produce carbon dioxide and water if there is a plentiful supply of oxygen. This is known as complete combustion. e.g. CH4 + 2O2 CO2 + 2H2O Write equations for the complete combustion of ...
Organic Nomenclature - Alkanes, Alkenes, Alkynes
... Naming Aldehydes (CH =O group) = -al ending An aldehyde is an organic molecule that has an oxygen atom doubly bonded to the terminal carbon of the backbone carbon chain. An aldehyde is named with the -al ending. Since the CHO must be on the terminal #1 carbon atom, the position of the CHO does not n ...
... Naming Aldehydes (CH =O group) = -al ending An aldehyde is an organic molecule that has an oxygen atom doubly bonded to the terminal carbon of the backbone carbon chain. An aldehyde is named with the -al ending. Since the CHO must be on the terminal #1 carbon atom, the position of the CHO does not n ...
CHE 312 Exam III Review Sheet - Saint Leo University Faculty
... Explain why an aromatic molecule like benzene reacts differently than the corresponding alkene (actually a –triene)? ...
... Explain why an aromatic molecule like benzene reacts differently than the corresponding alkene (actually a –triene)? ...
Discuss on Reactions of Alcohols
... temperatures, primary alcohols completely oxidize to form carboxylic acids. The common oxidizing agents used for these conversions are concentrated potassium permanganate or concentrated potassium dichromate. Following are several examples of this type of oxidation: ...
... temperatures, primary alcohols completely oxidize to form carboxylic acids. The common oxidizing agents used for these conversions are concentrated potassium permanganate or concentrated potassium dichromate. Following are several examples of this type of oxidation: ...
Unit 2: Nature`s Chemistry
... number this carbon chain to give lowest number to where double bond starts -> pent-2-ene indentify branches and indicate position with number in front of branch name 4-methylpent-2-ene ...
... number this carbon chain to give lowest number to where double bond starts -> pent-2-ene indentify branches and indicate position with number in front of branch name 4-methylpent-2-ene ...
슬라이드 1
... Conjugate addition to a,b-unsaturated esters can often be effected by copper catalyzed reaction with Grignard reagent. Other reactions, such as epoxide ring opening, can also be carried out under catalytic conditions. (Scheme 8.5) ...
... Conjugate addition to a,b-unsaturated esters can often be effected by copper catalyzed reaction with Grignard reagent. Other reactions, such as epoxide ring opening, can also be carried out under catalytic conditions. (Scheme 8.5) ...
Notetakers
... Formulas for organic compounds: empirical, molecular and structural Empirical: simplest whole number ratio of the atoms it contains. Example: empirical formula of ethane, C2H6 is CH3 Molecular: actual number of atoms of each present. It can be deduced if both the empirical formula and relative molec ...
... Formulas for organic compounds: empirical, molecular and structural Empirical: simplest whole number ratio of the atoms it contains. Example: empirical formula of ethane, C2H6 is CH3 Molecular: actual number of atoms of each present. It can be deduced if both the empirical formula and relative molec ...
Unit Two : Carbon Compounds
... 6. Position of functional group in alkenes, alkynes, alkanols, given by number within the name, eg. but-1-ene, butan-1-ol. 7. Number not needed for position of functional group in alkanoic acids. Always carbon number 1. ...
... 6. Position of functional group in alkenes, alkynes, alkanols, given by number within the name, eg. but-1-ene, butan-1-ol. 7. Number not needed for position of functional group in alkanoic acids. Always carbon number 1. ...
Lesson 19 - WordPress.com
... We can use oxidation reactions (and the products) to identify unlabelled primary, secondary and tertiary alcohols. Suggest a method to do so. ...
... We can use oxidation reactions (and the products) to identify unlabelled primary, secondary and tertiary alcohols. Suggest a method to do so. ...
organic revision nots
... UNIT: 10 Haloalkanes and Haloarenes. 1. Sulphuric acid is not used during the reaction of alcohols with KI. 2. Alkyl halides are generally not prepared in laboratory by free radical halogenations of alkanes. 3. The boiling points of alkyl halides decrease in the order: RI > RBr > RCl > RF. 4. Haloal ...
... UNIT: 10 Haloalkanes and Haloarenes. 1. Sulphuric acid is not used during the reaction of alcohols with KI. 2. Alkyl halides are generally not prepared in laboratory by free radical halogenations of alkanes. 3. The boiling points of alkyl halides decrease in the order: RI > RBr > RCl > RF. 4. Haloal ...
Organic Chem Functional Groups
... known as the trans isomer. (trans : from latin meaning "across" - as in transatlantic). In the other, the two chlorine atoms are locked on the same side of the double bond. This is know as the cis isomer. (cis : from latin ...
... known as the trans isomer. (trans : from latin meaning "across" - as in transatlantic). In the other, the two chlorine atoms are locked on the same side of the double bond. This is know as the cis isomer. (cis : from latin ...
04_01_03.html
... Overview of Chapter This chapter introduces chemical reactions and their mechanisms by focusing on two reactions that yield alkyl halides. (1) alcohol + hydrogen halide ROH + HX RX + H2O (2) alkane + halogen RH + X2 RX + HX Both are substitution reactions ...
... Overview of Chapter This chapter introduces chemical reactions and their mechanisms by focusing on two reactions that yield alkyl halides. (1) alcohol + hydrogen halide ROH + HX RX + H2O (2) alkane + halogen RH + X2 RX + HX Both are substitution reactions ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.