What are carboxylic acids?
... size are higher than alcohols. The higher boiling points of the carboxylic acids are still caused by hydrogen bonding. In a pure carboxylic acid, hydrogen bonding can occur between two molecules of acid ...
... size are higher than alcohols. The higher boiling points of the carboxylic acids are still caused by hydrogen bonding. In a pure carboxylic acid, hydrogen bonding can occur between two molecules of acid ...
ORGANIC CHEMISTRY
... deposits were formed is a mystery. But what is important is their size. It is estimated that the global ...
... deposits were formed is a mystery. But what is important is their size. It is estimated that the global ...
Chemistry for Changing Times 11th Edition Hill and Kolb
... Trichloromethane (chloroform) is also a solvent and at one time was used as an anesthetic. It is now considered hazardous. ...
... Trichloromethane (chloroform) is also a solvent and at one time was used as an anesthetic. It is now considered hazardous. ...
12SN-23-10 OBJECTIVE: Identify how alcohols are classified and
... This section explains how to distinguish among the carbonyl groups of aldehydes, ketones, carboxylic acids, and esters. It also describes the reactions of compounds that contain the carbonyl group. 1. A ______________________ consists of a carbon joined by a double bond to an oxygen atom. 2. What is ...
... This section explains how to distinguish among the carbonyl groups of aldehydes, ketones, carboxylic acids, and esters. It also describes the reactions of compounds that contain the carbonyl group. 1. A ______________________ consists of a carbon joined by a double bond to an oxygen atom. 2. What is ...
Organic Chemistry
... 7. use molecular geometries to determine intermolecular forces 8. interpret redox reactions 9. determine if reaction mechanisms fit experimental rate laws 10. use acid-base theory to predict reaction products and extent of reaction Expected Outcomes for Students: Upon completion of the course, the s ...
... 7. use molecular geometries to determine intermolecular forces 8. interpret redox reactions 9. determine if reaction mechanisms fit experimental rate laws 10. use acid-base theory to predict reaction products and extent of reaction Expected Outcomes for Students: Upon completion of the course, the s ...
Naming organic compounds
... Naming organic compounds The basic rules There are some general rules which you should remember when naming organic compounds. ...
... Naming organic compounds The basic rules There are some general rules which you should remember when naming organic compounds. ...
chemistry pretest - the Biology Scholars Program Wiki
... biochemistry course. Since for most of you it has been a while since you have taken these courses, I do not expect you to be up to speed with all of these questions. However, it is extremely important that you become comfortable with them as soon as possible and I would like to help you with that be ...
... biochemistry course. Since for most of you it has been a while since you have taken these courses, I do not expect you to be up to speed with all of these questions. However, it is extremely important that you become comfortable with them as soon as possible and I would like to help you with that be ...
Practice problems for week 8 PDF
... A meso compound is one that contains chiral centres but is achiral because of an intermal plane of symmetry. Thus, switching the R,S configuration does not produce a unique enantiomer but rather just another copy of the same compound. ...
... A meso compound is one that contains chiral centres but is achiral because of an intermal plane of symmetry. Thus, switching the R,S configuration does not produce a unique enantiomer but rather just another copy of the same compound. ...
File
... Ethanol is oxidised by this reaction. The final product of the oxidation reaction is ethanoic acid. CH3CH2OH + H2O → CH3COOH + 4H+ + 4e– 3CH3CH2OH + 3H2O → 3CH3COOH + 12H+ + 12e– ...
... Ethanol is oxidised by this reaction. The final product of the oxidation reaction is ethanoic acid. CH3CH2OH + H2O → CH3COOH + 4H+ + 4e– 3CH3CH2OH + 3H2O → 3CH3COOH + 12H+ + 12e– ...
Organic Chemistry Unit Test! /50
... 3. Look around you. There are plastics everywhere! How have organic chemists been able to do so much with such a basic starting material (ethene derivatives)? Explain why such variety in plastic properties is possible, and give an example of a plastic to illustrate one of your points (4 ...
... 3. Look around you. There are plastics everywhere! How have organic chemists been able to do so much with such a basic starting material (ethene derivatives)? Explain why such variety in plastic properties is possible, and give an example of a plastic to illustrate one of your points (4 ...
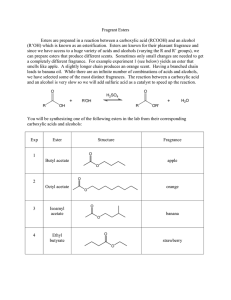
Fragrant Esters Esters are prepared in a reaction between a
... Esters are prepared in a reaction between a carboxylic acid (RCOOH) and an alcohol (R’OH) which is known as an esterification. Esters are known for their pleasant fragrance and since we have access to a huge variety of acids and alcohols (varying the R and R’ groups), we can prepare esters that prod ...
... Esters are prepared in a reaction between a carboxylic acid (RCOOH) and an alcohol (R’OH) which is known as an esterification. Esters are known for their pleasant fragrance and since we have access to a huge variety of acids and alcohols (varying the R and R’ groups), we can prepare esters that prod ...
CCCH110D Inorganic vs Organic NOTES
... HYDROCARBON REACTIONS: 1. Combustion Reactions: Hydrocarbons (alkanes, alkenes and alkynes) all undergo Combustion. In a combustion reaction, the hydrocarbon reacts with oxygen to form carbon dioxide and water. Refer to examples in Chapter 18. Hydrocarbon combustion reactions are highly exothermic – ...
... HYDROCARBON REACTIONS: 1. Combustion Reactions: Hydrocarbons (alkanes, alkenes and alkynes) all undergo Combustion. In a combustion reaction, the hydrocarbon reacts with oxygen to form carbon dioxide and water. Refer to examples in Chapter 18. Hydrocarbon combustion reactions are highly exothermic – ...
Organic Chemistry | Topic Notes
... Structural isomers are compounds with the same molecular formula but different structural formula. They differ in physical properties, e.g. boiling points. An example is Butane ------ Methylpropane ...
... Structural isomers are compounds with the same molecular formula but different structural formula. They differ in physical properties, e.g. boiling points. An example is Butane ------ Methylpropane ...
Chapter 12 Alcohols from Carbonyl Compounds: Oxidation
... Carbonyls react with nucleophiles π–orbital of formaldehyde The nucleophile adds to the δ+ carbon The π electrons shift to the oxygen The carbon becomes sp3 hybridized (tetrahedral!) Several chapters in Organic II For now, two nucleophiles that convert carbonyls to ...
... Carbonyls react with nucleophiles π–orbital of formaldehyde The nucleophile adds to the δ+ carbon The π electrons shift to the oxygen The carbon becomes sp3 hybridized (tetrahedral!) Several chapters in Organic II For now, two nucleophiles that convert carbonyls to ...
1 - Rosshall Academy
... Name the straight chain alkanes C1 to C8 from molecular formulae, shortened and full structural formulae. Write molecular formulae and draw full and shortened structural formulae given the names of straight-chain alkanes C1 to C8 Give the systematic names of branched chain alkanes from shortened and ...
... Name the straight chain alkanes C1 to C8 from molecular formulae, shortened and full structural formulae. Write molecular formulae and draw full and shortened structural formulae given the names of straight-chain alkanes C1 to C8 Give the systematic names of branched chain alkanes from shortened and ...
Alkene/Alkyne Addition Reactions
... obtained from the addition of an unsymmetrical reagent such as H-Br, H-Cl, or H-OH to an alkene or alkyne is the one obtained when the H atom of the reagent is added to the C atom of the multiple bond that already has the greater number of H atoms. “The rich get richer” ...
... obtained from the addition of an unsymmetrical reagent such as H-Br, H-Cl, or H-OH to an alkene or alkyne is the one obtained when the H atom of the reagent is added to the C atom of the multiple bond that already has the greater number of H atoms. “The rich get richer” ...
Chapter 3, Carbon, Dehydration and Hydrolysis
... The Synthesis and Breakdown of Polymers • Polymers are disassembled to monomers by hydrolysis, a reaction that is essentially the reverse of the dehydration reaction ...
... The Synthesis and Breakdown of Polymers • Polymers are disassembled to monomers by hydrolysis, a reaction that is essentially the reverse of the dehydration reaction ...
5.3 Organic Compounds
... Organic Compounds Organic compounds ALWAYS contain Carbon and ALMOST ALWAYS contain ...
... Organic Compounds Organic compounds ALWAYS contain Carbon and ALMOST ALWAYS contain ...
Experiment 4- Alkene
... Two mechanisms are possible for this dehydration reaction. The loss of H2O and H+ can occur in one step if the alcohol is primary: E2 mechanism (elimination bimolecular). Otherwise, if the alcohol is tertiary or has other structural features that can stabilize the corresponding carbocation, the elim ...
... Two mechanisms are possible for this dehydration reaction. The loss of H2O and H+ can occur in one step if the alcohol is primary: E2 mechanism (elimination bimolecular). Otherwise, if the alcohol is tertiary or has other structural features that can stabilize the corresponding carbocation, the elim ...
Exam 3 Review Sheet
... o Friedel-Crafts acylation: acid chloride, AlCl3 Can be followed by Wolff-Kishner (N2H4, KOH) or Clemmenson (Zn(Hg), HCl) reduction to eliminate oxygen. • Addition of the second and third groups o ortho/para vs. meta directors. Resonance structures, inductive effects. Activating the ring. • Sy ...
... o Friedel-Crafts acylation: acid chloride, AlCl3 Can be followed by Wolff-Kishner (N2H4, KOH) or Clemmenson (Zn(Hg), HCl) reduction to eliminate oxygen. • Addition of the second and third groups o ortho/para vs. meta directors. Resonance structures, inductive effects. Activating the ring. • Sy ...
Chapter 11 Introduction to Organic Chemistry Part 2
... 2 (a) Although both structures A and C are the staggered conformations of 2-methylbutane and more stable than structures B and D, which are eclipsed conformations, structure A will be the most stable conformation because all the methyl groups and hydrogen atoms are farther apart from each other than ...
... 2 (a) Although both structures A and C are the staggered conformations of 2-methylbutane and more stable than structures B and D, which are eclipsed conformations, structure A will be the most stable conformation because all the methyl groups and hydrogen atoms are farther apart from each other than ...
File
... • Boiling points higher than the corresponding alcohols • This is because carboxylic acids form dimers, where two carboxylic acid molecules are held together by two hydrogen bonds • This is possible due to polarity in both the C=O and O-H bonds in each carboxylic acid molecule • Methanoic acid is fo ...
... • Boiling points higher than the corresponding alcohols • This is because carboxylic acids form dimers, where two carboxylic acid molecules are held together by two hydrogen bonds • This is possible due to polarity in both the C=O and O-H bonds in each carboxylic acid molecule • Methanoic acid is fo ...
436
... _________________________________________________________ 1. Physical Examination : a) Physical State: ............................................................................. ...
... _________________________________________________________ 1. Physical Examination : a) Physical State: ............................................................................. ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.