pdf AP Chemistry Summer Assignment 2014 Dr. Hart`s classes
... Ions, solubility rules, common polyatomic elements, names of acids and common compounds, organic chemistry naming, colors of common ions, compounds and acid-base indicators. Expect quizzes addressing these topics periodically throughout the year until they become second nature. The first quiz, on al ...
... Ions, solubility rules, common polyatomic elements, names of acids and common compounds, organic chemistry naming, colors of common ions, compounds and acid-base indicators. Expect quizzes addressing these topics periodically throughout the year until they become second nature. The first quiz, on al ...
Balancing a Chemical Equation
... A chemical equation is balanced when the ions or atoms found on the reactant side of the equation equals that found on the ...
... A chemical equation is balanced when the ions or atoms found on the reactant side of the equation equals that found on the ...
Balancing a Chemical Equation
... A chemical equation is balanced when the ions or atoms found on the reactant side of the equation equals that found on the ...
... A chemical equation is balanced when the ions or atoms found on the reactant side of the equation equals that found on the ...
atomic number
... are fast moving electrons. Since electrons are lighter than helium atoms, they are able to penetrate further, through several feet of air, or several millimeters of plastic or less of very light metals. ...
... are fast moving electrons. Since electrons are lighter than helium atoms, they are able to penetrate further, through several feet of air, or several millimeters of plastic or less of very light metals. ...
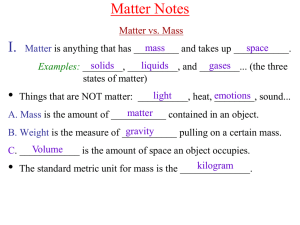
Chem A Week 2 Matter Notes
... B. In liquids, the particles move more __________ from place to place, and slide past each other in constant motion. However, they essentially contact remain in __________ with each other at all times as the slide around, so there is not much ...
... B. In liquids, the particles move more __________ from place to place, and slide past each other in constant motion. However, they essentially contact remain in __________ with each other at all times as the slide around, so there is not much ...
Balancing a Chemical Equation
... A chemical equation is balanced when the ions or atoms found on the reactant side of the equation equals that found on the ...
... A chemical equation is balanced when the ions or atoms found on the reactant side of the equation equals that found on the ...
Balancing chemical equations notes
... Chemical equations can be viewed as recipes for chemical reactions. They give a description of what chemicals are combined together and what chemicals are made when a reaction occurs. The law of conservation of mass says that matter can neither be created nor destroyed, and this requires that all ch ...
... Chemical equations can be viewed as recipes for chemical reactions. They give a description of what chemicals are combined together and what chemicals are made when a reaction occurs. The law of conservation of mass says that matter can neither be created nor destroyed, and this requires that all ch ...
Chapter #3
... There are N of the above equations, one for each element (atom type) in the reaction. Generally there are M coefficients to find using the N equations. Unfortunately, in most chemical equations, M > N. Usually, we have the case that M = N+1. Thus, we need to find one additional equation. One simple ...
... There are N of the above equations, one for each element (atom type) in the reaction. Generally there are M coefficients to find using the N equations. Unfortunately, in most chemical equations, M > N. Usually, we have the case that M = N+1. Thus, we need to find one additional equation. One simple ...
balancing eqns teacher
... A chemical equation is balanced when the ions or atoms found on the reactant side of the equation equals that found on the ...
... A chemical equation is balanced when the ions or atoms found on the reactant side of the equation equals that found on the ...
FirstSemesterReviewHonors
... Date _______ First Semester Review Chapters 1-12 ( no 9.4) , 22, 25 You may use the study guide on the final exam. You must provide all formulas where needed, since formulas will not be provided for you on the final. You should take at least 1 week to complete the material within the study guide. Ch ...
... Date _______ First Semester Review Chapters 1-12 ( no 9.4) , 22, 25 You may use the study guide on the final exam. You must provide all formulas where needed, since formulas will not be provided for you on the final. You should take at least 1 week to complete the material within the study guide. Ch ...
BERKELEY HEIGHTS PUBLIC SCHOOLS
... PHILOSOPHY/RATIONALE Chemistry Honors (#445) is designed to develop scientifically literate citizens who can use technology to investigate and explain the world around them. Using the scientific approach of observing, hypothesizing, testing, and analyzing, students explore the properties of matter a ...
... PHILOSOPHY/RATIONALE Chemistry Honors (#445) is designed to develop scientifically literate citizens who can use technology to investigate and explain the world around them. Using the scientific approach of observing, hypothesizing, testing, and analyzing, students explore the properties of matter a ...
Chapter 2 - Cloudfront.net
... identical intensive properties because every sample has the same composition. ...
... identical intensive properties because every sample has the same composition. ...
$doc.title
... with one oxygen atom to form one molecule of water. On the atomic scale, we never see an example of one and a half hydrogen atoms combining with an oxygen atom. This was one of the first observations of the early chemists who explored the properties of chemical elements. This observation is known as ...
... with one oxygen atom to form one molecule of water. On the atomic scale, we never see an example of one and a half hydrogen atoms combining with an oxygen atom. This was one of the first observations of the early chemists who explored the properties of chemical elements. This observation is known as ...
Intro to Chem
... sample has the same composition. Substance – Matter that has a uniform and definite composition. Physical property – a quality or condition of a substance that can be observed or measured without changing the substance’s composition. ...
... sample has the same composition. Substance – Matter that has a uniform and definite composition. Physical property – a quality or condition of a substance that can be observed or measured without changing the substance’s composition. ...
Summer - Honors Chemistry
... atoms of a given element have identical chemical properties, but not the same mass (isotopes). Elements can come in atomic form (e.g. Fe) or in molecular form (O2 or C60). About 80% of the elements are metals – these are to the left of the staircase on the periodic table (except for hydrogen – which ...
... atoms of a given element have identical chemical properties, but not the same mass (isotopes). Elements can come in atomic form (e.g. Fe) or in molecular form (O2 or C60). About 80% of the elements are metals – these are to the left of the staircase on the periodic table (except for hydrogen – which ...
History of chemistry

The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis to the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze.The protoscience of chemistry, alchemy, was unsuccessful in explaining the nature of matter and its transformations. However, by performing experiments and recording the results, alchemists set the stage for modern chemistry. The distinction began to emerge when a clear differentiation was made between chemistry and alchemy by Robert Boyle in his work The Sceptical Chymist (1661). While both alchemy and chemistry are concerned with matter and its transformations, chemists are seen as applying scientific method to their work.Chemistry is considered to have become an established science with the work of Antoine Lavoisier, who developed a law of conservation of mass that demanded careful measurement and quantitative observations of chemical phenomena. The history of chemistry is intertwined with the history of thermodynamics, especially through the work of Willard Gibbs.