Writing and Classifying Balanced Equations
... a. Write the skeleton equation to show each substance as it occurs in the diagrams. Compounds are shown with their atoms touching each other and have a subscript in their chemical formula representing the number of atoms bonded together. For example is NH3 b. Balance the equation using coefficients. ...
... a. Write the skeleton equation to show each substance as it occurs in the diagrams. Compounds are shown with their atoms touching each other and have a subscript in their chemical formula representing the number of atoms bonded together. For example is NH3 b. Balance the equation using coefficients. ...
Atoms and Elements: Are they Related?
... • Which parts of an atom make up the mass of the atom? • Elements are made up of? • The element lead is made up of what kind of atoms? ...
... • Which parts of an atom make up the mass of the atom? • Elements are made up of? • The element lead is made up of what kind of atoms? ...
Parallel Computing in Chemistry
... and to compute chemical properties therefrom. • Molecular dynamics is the attempt to simulate the motion of atoms and molecules in space over short (1-10ns) timespans. • There is actually some overlap between the two. ...
... and to compute chemical properties therefrom. • Molecular dynamics is the attempt to simulate the motion of atoms and molecules in space over short (1-10ns) timespans. • There is actually some overlap between the two. ...
Chemistry_in_Parallel_Computing_old
... and to compute chemical properties therefrom. • Molecular dynamics is the attempt to simulate the motion of atoms and molecules in space over short (1-10ns) timespans. • There is actually some overlap between the two. ...
... and to compute chemical properties therefrom. • Molecular dynamics is the attempt to simulate the motion of atoms and molecules in space over short (1-10ns) timespans. • There is actually some overlap between the two. ...
A.P. Chemistry Complexation Reactions
... (B) in a compound A single element must be more reactive to replace another element. ...
... (B) in a compound A single element must be more reactive to replace another element. ...
What are atoms? Notes - Riverdale Middle School
... Scientific law is a rule that describes a pattern in nature but does not try to explain why something happens. Example: newton’s laws of Motion Scientific law vs. Scientific theory Both are based on repeated observation and can be rejected or modified. A scientific law states that an event will occu ...
... Scientific law is a rule that describes a pattern in nature but does not try to explain why something happens. Example: newton’s laws of Motion Scientific law vs. Scientific theory Both are based on repeated observation and can be rejected or modified. A scientific law states that an event will occu ...
CHAPTER 2
... -Oxygen (O) is the power source of life on earth by combining with other substances, and has allotropes -Sulfur (S) (and even Selenium, Se) is fowl smelling, and S appears as allotropes -these elements are considered poisonous, but essential for human diets -Some variations is chemistries, but form ...
... -Oxygen (O) is the power source of life on earth by combining with other substances, and has allotropes -Sulfur (S) (and even Selenium, Se) is fowl smelling, and S appears as allotropes -these elements are considered poisonous, but essential for human diets -Some variations is chemistries, but form ...
The Periodic Table
... An atom isthe smallest the partnucleus of an are element Surrounding a series cloud like energy levelsproperties called shells that of has all the element’s or orbitals ...
... An atom isthe smallest the partnucleus of an are element Surrounding a series cloud like energy levelsproperties called shells that of has all the element’s or orbitals ...
What is Chemistry? Chemistry
... ______________________________: are the chemicals that begin or go into the reaction before it takes place. Chemical reaction: when the chemicals that go into the reaction (___________________________) change and new chemicals (______________________) are formed. When we write these “skeleton ...
... ______________________________: are the chemicals that begin or go into the reaction before it takes place. Chemical reaction: when the chemicals that go into the reaction (___________________________) change and new chemicals (______________________) are formed. When we write these “skeleton ...
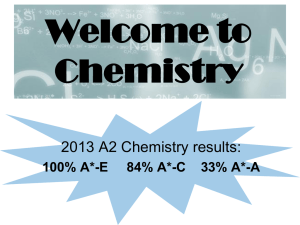
Welcome to Chemistry
... A numerate subject such as CHEMISTRY is useful for… • Accountancy/Business • Architecture • Law ...
... A numerate subject such as CHEMISTRY is useful for… • Accountancy/Business • Architecture • Law ...
Using mass to calculate molecular formula
... on the positive ions (cations) as there are on the negative ions (anions) and this determines the formula for that compound. Example: Sodium chloride, NaCl, 'table salt' ...
... on the positive ions (cations) as there are on the negative ions (anions) and this determines the formula for that compound. Example: Sodium chloride, NaCl, 'table salt' ...
Chapter 1 Reading Guide
... Guidelines for determining the number of significant figures in a measured quantity are: • The number of significant figures is the number of digits known with certainty plus one uncertain digit. (Example: 2.2405 g means we are sure the mass is 2.240 g but we are uncertain about the nearest 0.0001 g ...
... Guidelines for determining the number of significant figures in a measured quantity are: • The number of significant figures is the number of digits known with certainty plus one uncertain digit. (Example: 2.2405 g means we are sure the mass is 2.240 g but we are uncertain about the nearest 0.0001 g ...
Investigating Chemistry - Chemistry at Winthrop University
... Groups 13-16 are referred to by the first element or simply the group number. Group 17 is the Halogens. Group 18 is the Noble Gases. Elements 58-71 and 90-103 are called the Inner Transition Metals. ...
... Groups 13-16 are referred to by the first element or simply the group number. Group 17 is the Halogens. Group 18 is the Noble Gases. Elements 58-71 and 90-103 are called the Inner Transition Metals. ...
Topic 2
... Early in the 19th century John Dalton developed atomic theory. His theory explained the best available experimental data at that time. His theory has been modified since then with the discovery of other data, but his work was the initial ground ...
... Early in the 19th century John Dalton developed atomic theory. His theory explained the best available experimental data at that time. His theory has been modified since then with the discovery of other data, but his work was the initial ground ...
Periodic Trends & the Periodic Table
... • The elements in Groups 3 through 12 of the periodic table are called the transition elements. • All transition elements are metals. • Many transition metals can have more than one charge ...
... • The elements in Groups 3 through 12 of the periodic table are called the transition elements. • All transition elements are metals. • Many transition metals can have more than one charge ...
Unit 1: Matter and Energy HW Packet
... Part 8: Decide whether the following statements are TRUE or FALSE. 1. __________ Both elements and compounds are pure substances. 2. __________ A heterogeneous substance has at least two pure substances in it. 3. __________ Pure substances have variable composition. 4. __________ Compounds have vari ...
... Part 8: Decide whether the following statements are TRUE or FALSE. 1. __________ Both elements and compounds are pure substances. 2. __________ A heterogeneous substance has at least two pure substances in it. 3. __________ Pure substances have variable composition. 4. __________ Compounds have vari ...
Variation in Properties of Group II Compounds
... Each group of elements embodied in the periodic table has their own unique properties. As for group II elements, they are classified as one of the s-block elements, also named as alkaline earth metals. In this essay, the variation in properties of group II elements and their compounds are illustrate ...
... Each group of elements embodied in the periodic table has their own unique properties. As for group II elements, they are classified as one of the s-block elements, also named as alkaline earth metals. In this essay, the variation in properties of group II elements and their compounds are illustrate ...
Document
... • Many times it may seem that things we use disappear over time. For example, gasoline in the car. • Elements of gasoline are merely re-arranged through a chemical reaction. Gasoline CO2 and H2O • The number of each type of element and their masses remain unchanged (balanced) in a chemical reactio ...
... • Many times it may seem that things we use disappear over time. For example, gasoline in the car. • Elements of gasoline are merely re-arranged through a chemical reaction. Gasoline CO2 and H2O • The number of each type of element and their masses remain unchanged (balanced) in a chemical reactio ...
CHEMISTRY
... sum of the masses of the elements that make up the compound. • The ratio of the mass of each element to the total mass of the compound is a percentage called the percent by mass. ...
... sum of the masses of the elements that make up the compound. • The ratio of the mass of each element to the total mass of the compound is a percentage called the percent by mass. ...
Chemistry Academic v. 2016
... destroyed in ordinary chemical and physical changes; Law of Definite Proportions (Constant Composition) - A chemical compound always contains the same elements in exactly the same proportions by weight and mass; Rutherford’s Atomic Model- First modern concept of atomic structure; all of the positive ...
... destroyed in ordinary chemical and physical changes; Law of Definite Proportions (Constant Composition) - A chemical compound always contains the same elements in exactly the same proportions by weight and mass; Rutherford’s Atomic Model- First modern concept of atomic structure; all of the positive ...
History of chemistry

The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis to the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze.The protoscience of chemistry, alchemy, was unsuccessful in explaining the nature of matter and its transformations. However, by performing experiments and recording the results, alchemists set the stage for modern chemistry. The distinction began to emerge when a clear differentiation was made between chemistry and alchemy by Robert Boyle in his work The Sceptical Chymist (1661). While both alchemy and chemistry are concerned with matter and its transformations, chemists are seen as applying scientific method to their work.Chemistry is considered to have become an established science with the work of Antoine Lavoisier, who developed a law of conservation of mass that demanded careful measurement and quantitative observations of chemical phenomena. The history of chemistry is intertwined with the history of thermodynamics, especially through the work of Willard Gibbs.